Aakash Shingada has done it again with another smashing visual abstract.
Antibiotics and Kidney Stones, the visual abstract
Another smashing visual abstract by the NSMC crew. This one is by Sri Lekha Tummalapalli.
Apixaban or Warfarin in Atrial Fibrillation, the Visual Abstract
Sarah Gleeson has made a wonderful Visual Abstract for this week's NephJC
Law 1: When @Kidney_Boy learned about Bayesian Statistics
Law 1: A strong intuition is more powerful than a weak test
law 3: Mostly Wrong
Law 3: For every perfect medical experiment, there is a perfect human bias.
The brevity ofThe Laws of Medicine makes it more powerful. The book can be devoured in a single morning, but the reader will still be left profoundly impacted.
Dr. Siddhartha Mukherjee challenges the reader to go beyond contemplating the three laws that make up the book, but to conceive and open one's eyes to other Laws. Today, with an expanding data-driven world, we discount our humanity. From the author’s note:
“It’s easy to make perfect decisions with perfect information. Medicine asks you to make perfect decisions with imperfect information.”
This imperfect information is then processed through the imperfect spectacles of the human mind.
In 1954 Dr. Richard Asher wrote that we are men of action, but every action is, and ought to be, preceded by a certain amount of thought. We should carefully examine these thought processes as they can be faulty. Dr. He says that crooked or dishonest thinking can occur in medicine & classifies crooked thinking in medicine under therapeutics, statistics, causes, words, etc. He says that the concept that a statistical relationship between two things automatically implies a causal relationship between them is “perverse.” He points out, for example, that statistics would show the incidence of erythroblastosis foetalis is twenty times less common in children of opium smokers. However, this should not obviously lead to prescribing opium in the maternity ward. These types of causal relationships are among our human biases that have existed from well before Dr. Asher’s publication over sixty years ago, and are present in today’s medical practice as stated in Law 3.
Law 1: a strong intuition is much more powerful than a weak test, and Law 3 are closely related. Law 1 can be paraphrased as “every diagnostic challenge in medicine can be imagined as a probability game.” Probability is based on objective factors such as prior organ damage and testing, but also the physician’s history taking, instincts, and interpretation of the data. One of my favorite passages in the book is when Dr. Mukherjee quotes Dr. Bernie Fisher: “In God we trust. All others must bring data.” However, being human, our biases can change the lens through which all this data is examined. If the data collector is flawed, does that not mean his accruement, analysis, and interpretation of the information is flawed? How do we account for this? Dr. Mukherjee says that reading a study inherently introduces human perception, arbitration, and interpretation – and hence involves bias. Bias is not only limited to research and those reading and analyzing the study. What bias can the subjects of the study introduce? One should consider the possibility of the Hawthorne Effect- when a human knows they are being observed, behavior changes. How do we account for these biases?
What about making medical decisions? Often complex decisions are made using mental heuristics and shortcuts to reduce complexity. These shortcuts develop over time with experience and increased knowledge in the field. Biases however, particularly cognitive biases, can easily creep in. While in medical school, I would hear of a symptom, or learn of a treatment, and try to apply this to all similar clinical situations (anchoring bias). Even experienced physicians are susceptible to similar biases. How often do we ask what is the data for established treatments? Sodium polystyrene sulfonate (Kayexelate) was approved based on two studies that barely deserve the name studies (no controls, confounders such as low K diet, diuretics, and other drugs that lower potassium). In 2011, the FDA issued a warning that Kayexelate was associated with colonic necrosis. For over fifty years Kayexelate was used without much consideration until there were significant complications in patients. Did the fact that Kayexalate was approved and was an old drug allow this complication to be overlooked for so long?
This brings me to the practice of evidence based medicine (EBM) and guidelines. In 1996 Dr. Sackett defined EBM as “the conscientious, explicit, and judicious use of current best evidence in making decisions involving the care of an individual patient. It integrates individual clinical expertise with the best available clinical evidence from systematic research.” How does this evidence come about? One thinks of a clinical question and the important outcomes to measure. The results are analyzed, and if found to be “good,” used in evidence based care. This evidence is often used to deliver a doctor-defined patient agenda. A medical encounter takes place and the health care provider focuses on what choices need to be made and carried out for the patient.
Has the research and medical decisions incorporated the patient? Did the research take into account the experience of their subjects? These questions are at the core of the movement to expand Patient Reported Outcome Measures (PROMs) and Patient Reported Experience Measures (PREMs) in our research agendas. We also must remember that EBM looks at the care of populations as opposed to individual patients. Dr. Shyaan Goh, an Orthopedic Surgeon from Australia, wrote to the British Medical Journal that the clinicians are the problem when EBM adversely affects clinical judgment. The clinician might not account for the quality or applicability of the evidence; they might not understand the rationale behind a guideline and not properly observe the guideline.
The question asked throughout this commentary is how do we account for these biases? How do we guard ourselves from allowing our own human instincts that may lead us astray? I think it is imperative to focus on the central figure of the story, the patient, not the health care provider. We have to take into account the individual who is experiencing the illness- what are their goals and expectations? A patient is not just a series of questions and tests.
As for the clinicians themselves, what other advice might be helpful? Well, Dr. Asher implored us to keep on the path of straight-thinking, regardless of the destination, to avoid crooked thinking. He quoted Rudyard Kipling’s Elephant’s Child to provide us with “helpers” to achieve this end:
“I keep six honest serving-men,
Their names are What and Why and When
and How and Where and Who.”
Maybe we should consider having these six helpers at our side aiding us to “hunt” and bring our biases to the forefront. Then, as Dr. Mukherjee says, we can confront bias head-on and incorporate it into the very definition of medicine.
Commentary by Beje Thomas, Nephrologist
NSMC intern, class of 2018
Additional Reading:
- Asher R. Straight and crooked thinking in medicine. BMJ 1954;2:460.
- Lehman Richard. Siddhartha Mukherjee’s three laws of medicine. BMJ 2015; 351 :h6708
- Accad, M. and D. Francis. "Does Evidence Based Medicine Adversely Affect Clinical Judgment?" BMJ (Clinical Research Ed.) 362,
- Greenhalgh T et al. Six ‘biases’ against patients and careers in evidence-based medicine. BMC Med 2015;13:200
- Goh S. The Problem with Evidence Based Medicine is really the Clinicians. (Letter to the editor). BMJ. 2018 July 18
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–72. doi: 10.1136/bmj.312.7023.71.
Law 3: For every perfect medical experiment, there is a perfect human bias.
It is indeed nice to have three laws - recall Newton, and perhaps more famously, Asimov’s three laws of Robotics. It's pithy, epigrammatic and attempts to ground us. The laws teach us humility even as research and innovation reach ever upwards. So what does Mukherjee mean by this third law?
He starts off with his fellowship in Oncology. The Human Genome Project had its great moment, and though the term ‘precision medicine’ had not even made it past a synapse, the oncology world was dealing with precisely engineered, monoclonal antibodies with fabulously unheard of outcomes. Following on the success of the VEGF inhibitor Imatinib (Gleevec), there was another ‘cousin’ that Mukherjee and his cohort of fellows were seeing used. The fellows saw dramatic positive results in their patients - but somehow, paradoxically, and in stark contrast, the actual clinical trial showed little benefit. How did this happen? Selection bias struck the fellows. They were handed patients from graduating fellows who had the most ‘educational value’ aka the patients doing well. On the other hand, the patients who did not do well were handed back to the attending physician. Like the parable of the broken window, one has to be careful about what is not seen. The patients who are lost to follow up may be lost because they are too sick to come back. Though this was not a perfect experiment that Mukherjee describes - it does illustrate a common enough bias - bring it up the next time an experienced colleague starts off with an ‘in my experience…’ to counter your meticulously gathered data. Vinay Prasad explains the responder bias, all too common in oncology, in a nice tweetorial here.
Another example cited by Mukherjee is the radical mastectomy - Halsted’s procedure, championed by a famous Hopkins surgeon in 1882. In a clever piece of naming, the radical makes one imagine that the roots of cancer have been eradicated, and it took nearly a century before the futility of the approach was revealed by randomized controlled trial. A clever study from Giovannucci showed an example of recall bias. In women with breast cancer, the diet history taken after the cancer diagnosis seemed to suggest that high fat intake was associated with cancer. However, a dietary history taken a decade prior to the diagnosis of cancer, from the same women, showed no similar association. The cancer diagnosis creates false memories. Food questionnaires, forgive the pun, should be taken with a pinch of salt.
But these are all epidemiological studies. Surely randomized controlled trials are not biased. The entire rationale is to prevent these kind of confounding, selection bias - or information biases to creep in. However, the trial methods do count. Though Mukherjee doesn’t go in to those aspects, blinding, allocation concealment, proper randomization are but some additional features of trial quality which can bias even the best laid plans. Check out the Cochrane risk of bias tool, which explains a few of these in detail. There is more to this of course. Should one change practice on the basis of a single small trial? Enter publication bais - or the shelf drawer (full of unpublished negative trials) bias. How about the more important issue of generalizability, or external validity? Does a psychology study of WEIRD individuals apply to all humanity? Surely not. Men and women are biologically different - but not for all conditions and surely not in response to all therapies. The need to do trials in all subpopulations is sometimes carried too far, however, in denying effective therapies to dialysis patients. Just because a trial has been done in the general population doesn’t mean the therapy will not work in dialysis patients. Generalizability should not be an excuse to practice renalism.
So, Mukherjee wants us to be bias hunters, on the look out for biases in every study. Eternal vigilance is always necessary.
Summary by Swapnil Hiremath, Ottawa
Law 2. Outliers by Mukherjee, not Gladwell.
“Single patient anecdotes are often dismissed…But here, exactly such an anecdote had turned out to be a portal to a new scientific direction.”
In The Laws of Medicine by Dr. Siddhartha Mukherjee, he describes a researcher who noticed an outlier in a clinical trial of a new drug for bladder cancer who responded to the treatment when others had not. Through genetic studies, they were able to find specific mutations in the tumor that conferred a higher likelihood of response, thus opening new avenues for therapy. He argues that you should pay attention to those outliers as much (or more) as you do the rest of the population, because they probably have something very valuable to teach us. I couldn’t agree more.
Pediatric nephrology is full of anecdotes that have informed nephrologists about the most basic kidney physiology. Congenital nephrotic syndrome was first described in the 1950s by Finnish nephrologists. While nephrologists were very familiar with typical minimal change nephrotic syndrome, these extremely rare children were different. They presented shortly after birth with massive proteinuria and died usually within the first year of life from infection. With improvements in supportive care, these children survived to kidney transplantation, however they still didn’t respond to our now standard treatment of steroids for nephrotic syndrome. It wasn’t until 1998, that the gene mutation responsible for this disorder was identified as nephrin (NPHS1), a slit diaphragm protein required for maintenance of the glomerular filtration barrier. This opened up a decade of fundamental research into the role of the podocyte in glomerular disease and helped to inform us about how the glomerular filtration barrier works.
In a less glomerulocentric view of the world (yes, I suppose there is such a thing), children with an unusual form of autosomal dominant hypertension were identified in the 1960s who had early onset disease with hypokalemia, suppressed renin and aldosterone, and responsiveness to triamterene but not spironolactone or dexamethasone. What on earth was going on in the tubule? It was long suspected that there was a defect in a sodium channel leading to salt and water retention, however the exact cause was elusive. Finally, in 1994, mutations in genes encoding the epithelial sodium channel (ENaC) [SCNN1B, SCNN1G] were found to be the cause of Liddle syndrome, opening new doors into research on blood pressure homeostasis and sodium handling in the kidney.
Genetic kidney diseases are one of my favorite parts about pediatric nephrology. You can learn so much about normal physiology when just one little thing goes wrong. But, you have to recognize the outlier and start to think about how they got there. Is this a thing of the past? Have all the genetic causes of disease been discovered already? Of course not. Keep your eyes open for that zebra.
Commentary by Michelle Rheault, Minneapolis
Law 2. Normals teach us rules; Outliers teach us laws
I search for patterns, always have. The logic of symptomatology is Glorious. I suspect this character trait is common in nephrologists. But revelling in the symptoms and signs that lead to a diagnosis and the investigative algorithm that refines the differential into the definite, has meant that I focus on what Siddhartha terms “inliers”. As I seek to make a patient “fit” my pre-conceived pathogenic model, I run the risk of missing a deeper truth. Brahe concentrated on the inliers and modelled the movement of the planets into concentric circles, frustrated that Mars wouldn’t fit this model. Kepler used the movement of the outlier, Mars, to reveal that all the planets orbit the sun in concentric ellipses. By concentrating on the range of normalcy we can only create rules, whereas “outliers” allow access to deeper laws.
Each outlier represents an opportunity to refine our understanding of illness. Asking why one patient in a thousand has responded to a drug, can reveal new disease pathways. David Solit used this approach to investigate why one woman’s advanced bladder cancer had a spectacular response to everolimus, while the drug appeared ineffective for the cohort as a whole. Sequencing that woman’s tumour showed multiple mutations, most interestingly in TSC1 and NF2, suggesting that these genes modulated the response to everolimus. The group went on to sequenced the same genes in the larger cohort and were able to segregate the group into responders and non-responders by the mutation in the TSC1 gene. In doing so they opened up new lines of enquiry and broadened the understanding of everolimus, bladder cancer, tuberous sclerosis and neurofibromatosis.
Through seeking to understand the outlier they opened up a deeper understanding of disease.
Now that case reports have fallen out of fashion, becoming almost impossible to publish and dismissed as anecdote, where are we to find our outliers? One option is to conduct “outlier rounds” as Siddhartha suggests, another is to seek out case report posters at conferences. But a further powerful option is to maintain an open dialogue with colleagues, whether in person or online, to tell our stories and to ask the deeper questions.
Cathy Quinlain
The Laws of Medicine: The Introduction
Before Siddhartha Mukherjee goes into the three laws of medicine he describes the origin of his search for the laws. It is a compelling read. Some of the truths he uncovers in these early pages are especially compelling. How about this gem from the Authors Note:
“It’s easy to make perfect decisions with perfect information. Medicine asks you to make perfect decisions with imperfect information.”
This is what makes doctoring so difficult. Even when the stakes are highest and lives are on the line we must make decisions with imperfect information. It is this stress that leads doctors to “overuse” radiology and laboratory services. We are always trying to perfect our information in the hope it will make our decisions “perfect.”
In describing how he started his search for the laws of medicine he tells the story of starting a medicine residency “in Boston.” He had a q3 call schedule. I had no idea those still existed in internal medicine in 1999. But I guess things move slowly in Boston. He does a wonderful job of describing the numbness that can come from a grueling residency,
”I ran through a park by night, and through friends by day”
His saving grace and only companion was a book by Lewis Thomas. Thomas was a medical resident in the 1930’s and described his experience of medicine in a slim paperback collection of essays titled, The Youngest Science.
Mukherjee describes medicine in the 30’s as emerging from a dark ages of quack cures that was followed by a period of therapeutic nihilism where the quackery was halted and physicians were limited to just documenting, classifying, and describing illness. In the 30’s physicians began to interpret the carefully collected observations and develop new therapies.
Mukherjee became drive to answer a seemingly simple question,
Is medicine a science?
Obviously, it was scientific in that it used techniques that were derived from phenomenally sophisticated science. But for medicine to be a science it had to have its own laws, independent from biology and pharmacology. If Medicine is a science it had to have laws, and so what are they? In his own words,
But does the “youngest science” have laws? It seems like an odd preoccupation now, but I spent much of my medical residency seeking the laws of medicine. The criteria were simple: a “law” had to distill some universal guiding principle of medicine into a statement of truth. The law could not be borrowed from biology or chemistry; it had to be specific to the practice of medicine...
...My search for the laws was not an attempt to codify or reduce the discipline into grand universals. Rather, I imagined them as guiding rules that a young doctor might teach himself as he navigates a profession that seems, at first glance, overwhelmingly unnavigable.
This introduction is beautifully written, full of fascinating stories and successfully reminds the reader how young the science of medicine is. We literally have patients in the hospital who are older than the science of medicine.
This introduction also brilliantly sets up the remainder of the book. I have played this introduction to the resident teams I lead and they both relate and are intrigued.
Summary written by Joel Topf, Detroit
Underpowered or Negative? A closer look at not-so-positive trial results
Visual Abstract for RELIEF
Here is the visual abstract for tonight's chat. By Laura Slattery. Eat it up, but with just a small amount of water.
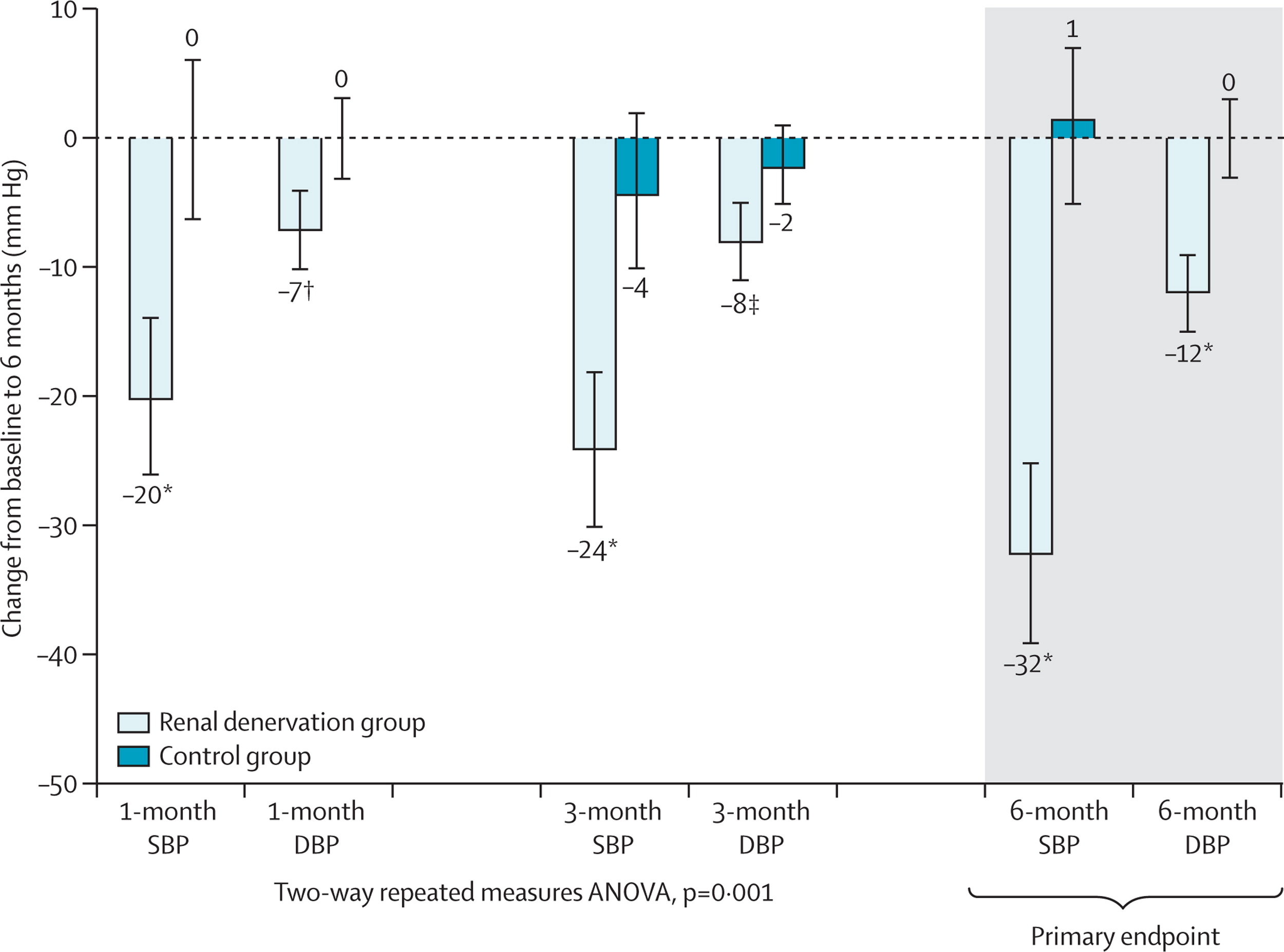
RADIANCE HTN SOLO: the Visual Abstract
The visual abstract for the RADIANCE HTN SOLO trial, again from Angel Ortiz
SPYRAL HTN ON: Visual Abstract
Another great visual abstract from Angel Ortiz, this one on the SPYRAL HTN ON trial.
SPYRAL HTN OFF: The Visual Abstract
NSMC intern Angel Ortiz has hit this one out of the park - he's made visual abstracts for all the three RDN trials we are discussing.
Check out the visual abstract of SPYRAL HTN OFF
RADIANCE SOLO: Denervation with a Difference?
SPYRAL ON: Renal Denervation in Patients ON Medications
SPYRAL OFF: Can Renal Denervation Reduce Blood Pressure in Hypertension?
Renal Denervation: the Story so far...
The Sympathetic System and Blood Pressure
We know that the sympathetic nervous system plays a role in blood pressure regulation. A long long time ago, when we did not have many - or any - blood pressure lowering medications - especially safe ones, options considered were colloidal sulphur and sodium thiocyanate.
In the early years of hypertension, some thought that elevated blood pressure was a homeostatic response to decreased tissue perfusion. In renal failure, high blood pressure was supposed to be required to excrete larger amounts of urea despite fewer nephrons. See Ludwig Traube in 1856.
One of the few options to lower really high blood pressure was to perform a dorsolumbar sympathetectomy, first hypothesized by Brüning in 1923. After a few unsuccessful attempts, and some on serendipitious observations of the effects of spinal anesthesia, Adson did a bilateral ventral rhizotomy, from the T6 to L2 level in a young woman. Her blood pressure fell from 250/180 to 170/120 mm Hg. A subsequent case series, showed consistently lower blood pressure - truly & spectacularly so. The amazing improvement in blood pressure was accompanied by just-as-impressive side effects. Apart from surgical morbidity and occasional mortality, there was loss of sensation, paralytic ileus, problems with ejaculation, and loss of sweating. And then, a few years later, blood pressure lowering medications started becoming available, such as reserpine in 1949, and in the mid 60's Sir James Black developed propranolol. With the development of calcium channel blockers in the 1970s and captopril in the 1980s there was no looking back. Now A-C-D are established as first line therapy on the basis of safe and effective blood pressure lowering with beneficial effect on cardiovascular outcomes. The hot hypertension debates have moved on to what level of blood pressure lowering (120 or 130 or 140, not 250!) and not on use of surgical procedures and whether blood pressure should be lowered at all.
Renal Denervation Development
However, a minority of patients with hypertension have blood pressure that does not respond to first line agents (typically the A-C-D combination) mentioned above. Though second line agents are aplenty, they have higher rates of adverse events, as well as little data on long-term, hard-outcomes. Hence this area is still considered ripe for testing newer interventions, such as new drugs and novel strategies. One of the most promising entrants here was the development of percutaneous renal denervation, first reported in 2009, from Murray Esler, Markus Schlaich and colleagues from the Baker Institute in Melbourne. The patient they reported had severe resistant hypertension with end organ damage, underwent percutaneous radiofrequency ablation of the sympathetic nerves around both of the renal arteries, resulting in a decrease in kidney, as well as whole body norepinephrine spillover and muscle sympathetic activity. This was accompanied by an impressive 20/17 mm Hg drop in blood pressure - not as much as with dorsolumbar surgical sympathectomy, but impressive nevertheless given that this was on a background of multiple medications.
From Schlaich et al, NEJM 2009: the first case report in the modern era of RDN
The group quickly followed up with two large prospective trials. Symplicity HTN-1 was a single arm prospective trial, which enrolled 50 patients with resistant hypertension, defined as an office systolic BP of 160 or more on 3 or more drugs. It demonstrated a drop of 14/10 mm Hg at one month, which seemed to progressively increase to 27/17 by 6 months of follow up.
From Krum et al, Lancet 2009: Main result of Symplicity HTN-2
This proof-of-concept trial was followed by the Symplicity HTN-2, this was an open-label, randomized controlled trial in about 100 patients with resistant hypertension. The results showed a decrease in BP of 20/7 at 1 month and 32/12 at 6 months. Renal denervation (RDN) had arrived! CE approval in Europe followed soon after and rates of renal denervation soared. The company that developed RDN (Ardian) was bought by the device behemoth Medtronic for $800 million. Other device companies with different catheters entered the fray. Hypertension was cool again. Until Symplicity HTN-3.
From Esler et al, Lancet 2010: Main results of Symplicity HTN-2
The Crash
Despite CE approval in Europe, RDN was not approved in the US. The FDA insisted on a rigorous trial undertaken in the US. This was Symplicity HTN-3 (S-3). The catheter and technique were the same ones, now from Medtronic. But the trial was different in many salient ways. S-1 and S-2 included patients with an office BP > 160 mm Hg, and the outcomes were office based blood pressure changes. In S-3, the trialists not only required office BP > 160 on 3 or more drugs at maximal dosage, but also 2 weeks of home BP measurements followed by a repeat office visit to measure BP. They all also needed 24-hour ambulatory blood pressure monitoring (ABPM, see #NephMadness post on why this is important), showing daytime systolic BP > 135. This requirement resulted in the trialists enrolling 535 from 1441 potentially eligible patients. Most importantly perhaps, S-3 had a control arm in which the patient underwent renal angiography, with a ‘sham’ RDN procedure. Thus patients were blinded - and so were the trial personnel measuring blood pressure. The results of S-3 came as a shock to the hypertension community. They demonstrated no difference between RDN and control (ie sham RDN) in either office or ABPM. Many explanations were bandied about (see the emergency #NephJC session details and hangout video for more). But RDN was dead, as far as North America and many other places in the world were concerned.
From Bhatt et al, NEJM 2014, Main result of Symplicity HTN-3
Why did S-3 differ from previous RDN trials?
In the immediate aftermath of S-3, some of the key reasons that were thrown around were: heterogeneity of the population (there was a suggestion of less effect in the African-American population versus others) and the completeness of the RDN (the number of ‘notches’ corresponded loosely with the BP lowering). Medtronic developed a newer catheter system (‘Spyral’) which could do radioablation at different points both longitudinally and circumferentially, thus reducing the issue of operator expertise.
Design of the newer SPYRAL catheter system
With respect to the efficacy of actual nerve ablation, it is indeed important to note that there is, yet, no physiological test or biomarker to assess whether the RDN was successful. The norepinephrine spillover or muscle sympathetic nerve activity testing are accurate, but time consuming and only used as research tools. Blood pressure lowering is a physiological test, but it is very noisy.
On the noisiness of blood pressure, one of the fascinating analysis that came from S-3 was from Darrel Francis’s group (yes the same Professor of ‘tweetorial’ fame). A few months before S-3, they predicted that the effect in a sham-controlled trial would be less than in previous trials. S-3, of course, didn’t show just a smaller effect, it reported no effect of RDN compared to sham. So Francis and his smart fellows (James Howard) conducted a further quantification of the three biases that they measured as being important to understand in this area.
Regression to the mean: First described by Francis Galton (‘regression to mediocrity’) this is an important aspect of a messy measurement like blood pressure. Selecting individuals with very high values and repeating another measure (as was done in the early RDN studies) is almost guaranteed to provide lower values - whether patients undergo RDN or not. Repeated measures, to confirm that the elevated blood pressure is truly elevated (say, with 2 weeks of home BP, or with ABPM), help reduce this - and a control group almost solves this. However, in the RDN studies, there was not much of a difference between controlled and uncontrolled studies - and even with unblinded controls who were randomized.
The second important bias then is information bias: in this case measurement bias. Observers who were unblinded taking BP measurements can be, ahem, biased, if they know whether the patient had or did not have RDN. Hence the use of automated measures (such as ABPM) reduces the effect size.
Lastly, blinding - which was a key aspect that was different between the two RCTs (S-2 and S-3). How would this have a different effect compared to unblinded controls? After all, BP - especially with ABPM, is an objective measure? However, knowing that one has had RDN may change behaviour - maybe patients become more adherent to their medication. Perhaps even the elusive placebo effect does truly exist in hypertension.
From Howard et al, Circulation 2016. Relationship between trial design and the reductions in office and ambulatory blood pressures. Each data point represents a trial. The area of the data point is proportional to the trial size. Red diamonds indicate the meta-analyzed estimate of the effect size for each trial design
Apart from these three aspects, it was also noted - from the same group - that variance in BP trials was higher when greater number of blood pressure medications were prescribed.
From Howard et al, J Hypertension 2015. Visit-to-visit variance in blood pressure within individuals increases with the number of medications prescribed. Sample size required for a trial is linearly dependent on visit-to-visit variance, the square of the visit-to-visit SDΔ, because the sample size required for a trial is linearly dependent on this.
Among the recommendations provided to overcome this problem were:
Solution 1: conduct renal denervation trials in patients on no medications (note this is exactly opposite of what was being done so far: testing RDN in patients with resistant hypertension)
Solution 2: continue prior medications, but pause medication for a limited period prior to baseline and final pressure measurements
Solution 3: directly observed antihypertensive therapy with a stable regimen
Based on this, in late 2014, a think tank comprised of representatives from the FDA, along with the NHLBI and the American Society of Hypertension came together and published some guidance for device therapy trials in hypertension, which was followed closely in the three trials we will be discussing at #NephJC this week.
Read so far? Now please go ahead and check out summaries of
Also check out the excellent Visual Abstracts from Angel Ortiz
Lastly, to prepare for the chat, see the summary of all three trials - and some discussion points.
Post by Swapnil Hiremath, Ottawa