#NephJC Chat
Tuesday June 6th 9 pm eastern
Wednesday June 7th 8 pm BST, 12 noon Pacific
J Clin Invest. 2017 May 1;127(5):1932-1943. doi: 10.1172/JCI88530. Epub 2017 Apr 17.
Increased salt consumption induces body water conservation and decreases fluid intake.
Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, Kopp C, Pedchenko T, Zhang Y, Beck L, Johannes B, Marton A, Müller DN, Rauh M, Luft FC, Titze J.
PMID: 28414302 Available for Free at JCI: Full Text
Accompanying Editorial (gated)
Introduction
The sodium physiology that you were taught, the physiology Edelman, is under assault. In fact everything we know about sodium physiology is crumbling under the weight of imaginative research and novel techniques. The first I learned about the assault was in Ingelfinger's 2015 sodium review in the NEJM:
When it became known that much of the sodium in the body is bound to bone, cartilage, and connective tissue, it was hypothesized that these tissues could serve as sodium reservoirs, taking up or releasing sodium in response to the needs of the body. Despite early evidence supporting the concept of a sodium reservoir, this theory lost favor and was not pursued for half a century. However, the past decade has seen renewed interest in stored sodium.11 In patients who consume high-salt diets, sodium can accumulate in the body, seemingly disappearing without a change in the plasma sodium concentration, body weight, or extracellular fluid volume. Sodium, potassium, and water balance do not always account for changes in the plasma sodium concentration during recovery from hyponatremia. Proteoglycans in skin serve as a sodium reservoir, and the number of negative charges available to bind sodium varies in response to the sodium concentration of interstitial tissue. In experiments in rats, chronic hyponatremia has been shown to be a more potent cause of osteopenia than vitamin D deficiency, and loss of sodium from bone exceeded the loss of calcium from bone. The activity of osteoclasts is increased in chronic hyponatremia owing to a direct effect of sodium and possibly vasopressin on these cells.
In case you TL;DR'd it, here is the essential renal heresy
“Sodium, potassium, and water balance do not always account for changes in the plasma sodium concentration”
As if millions of voices suddenly cried out in terror and were suddenly silenced. I fear something terrible has happened.
This week's NephJC continues the assault and you know the walls of knowledge have been breached when the New York Times runs with this headline:
It is a good article with quotes by #NephMadness Blue Ribbon Panel Member, Melanie Hoenig
The primary study question investigates the relationship of sodium intake to urine volume. The dogma is that increased sodium intake leads to increased thirst which then stimulates fluid intake. This is a central tenet for one of the theories explaining the obesity epidemic: increased dietary sodium stimulates thirst leading to increased consumption of sugar-sweetened beverages and thus increased obesity.
However previous work had not been able to find a relationship between sodium intake and urine volume.
Methods: We are going to Mars
The study used two simulated Mars missions for the study. Mars105 and Mars520 had 12 men in simulated space flight for 105 days and 520 days respectively. The participants were kept in a hermetically sealed environment while they lived and worked like cosmonauts. Environmental factors were kept constant.
During the Mars105 study salt intake was sequentially adjusted from 12 grams a day to 9 g/d to 6 g/d. During the Mars520 the salt intake was was adjusted from 12 g/d to 9 g/d to 6 g/d and then back to 12 g/d. The article plays a little loose with the terms salt and sodium and in fact at times confuses them. Conversion table:
Each salt level was maintained for 29 days. Calories and other minerals were fixed. Patients had unrestricted access to water and beverages. Fluid and sodium intake along with urine output were all rigorously tracked. (They measured urine output volumetrically, just like your hospital does, and gravimetrically, which is JCI-speak for "by weight")
The subjects went to 24-hour urine school. They had four 1-hour classroom sessions to teach the cosmonauts how to collect the 24-hour urine samples. For comparison I get 5 hours divided into two sessions (one lecture and one flipped classroom) to teach second year medical students everything they need to know about sodium water balance. The investigators monitored compliance and kicked out the losers:
We excluded subjects from analysis when their weekly urinary sodium recovery was repeatedly less than 80% of sodium intake or when the subjects did not adhere to our daily menu plans. Only this strict focus on experimental accuracy allowed us to implement a long- term balance approach. Because 2 subjects did not comply with these criteria, we had to exclude them from further analysis.
When they say 24-hour urine, they mean 24-hour urine.
In addition to sodium intake and excretion, fluid intake and excretion, the investigators also recorded serial cortisone, and aldosterone excretion; urine Na+, K+, and urea concentration; and urine osmolality.
Results
So the primary assessments were on urine sodium and urine volume on the three different salt diets.
Start with panels F and G. They show a straight forward increase in urine sodium over 24 hours as the diet goes from 6 to 9 to 12 grams of salt a day. Easy. Panels B and C show decreasing water intake as dietary salt intake rises. That makes no sense. Panels D and E look at urine volume across dietary salt intake. No clear pattern emerged, with the lowest urine volume with the middle salt diet. The green lines look at the measured variable not by dietary intake but by 24-hour urine sodium. The authors presented data that dietary sodium in does not equal urinary sodium out in a neat 24-hour cycle. That people hold on to sodium and that the diuresis can be delayed for days. To account this temporal discrepancy they presented the data using tertiles of urine sodium and then the urine volume makes more sense with increasing urine output with increased urine sodium.
In trying to explain why water intake went down with increasing water excretion the investigators looked to urine concentration and the concept of free water clearance. Urine that is hyperosmotic compared to plasma results in a negative free water clearance (FWC). Meaning that when the patient generates urine they are creating water that is added back to the circulation.
The investigators showed that with higher dietary salt intake urine concentration rose and so did free water generation. To the point that at 12 grams of salt a day patients were generating a liter of free water everyday. This endogenuos water creation suppressed thirst.
The investigators then looked at what was driving the increase in urine osmolality. They found that the rise in urine osmolality was almost entirely from increased sodium and an associated anion, while urine urea actually fell. The authors speculate that the urea was accumulated in the renal medullary interstitium to allow ADH-driven concentration of the urine.
Note the fall in urine urea as the salt intake rises
There was a stepwise increase in serum and 24-hour urinary aldosterone as patients went from 6 g to 12 g salt diets. Additionally, while salt was fixed the investigators noted a cyclical rise and fall of urine aldosterone and urine cortisol.
However, as reported earlier (11), UAldoV and UCortisoneV showed additional rhythmical half-weekly and weekly patterns of change that were independent of salt intake.
So the authors stratified their findings across high, medium and low aldosterone and cortisol levels for each dietary salt intake. They noted the following findings:
Increased aldosterone was associated with less urine volume and more water intake. So total body water (and body weight) went up
Increased cortisol was associated with increased urine volume without increased water intake. There was also no loss of body weight, suggesting that this increased urine volume was endogenously created water.
Rhythmical glucocorticoid release was therefore linked to increased diuresis in the context of the urine dilution mechanism by increasing FWC
The author then projected the combined glucocorticoid and mineralocorticoid effects in response to changes in dietary salt challenge and estimated that a 6 gram increase in dietary salt would increase urine volume by 358 ml/day and decrease oral intake of water by 60 ml.
There is also an accompanying paper, from the same group, which was done in mice, and which supports these conclusions.
Discussion
There are a few key lines in the discussion that I want to call out. First is this gem to try to wrap your brain around:
the excretion of a Na+ and Cl– osmolyte surplus of 201 ± 8 mmol/d at the 12-g/d salt intake level eventually reduced fluid intake, even though urine volume was increased.
Try to square that with your classical nephrology education, increased sodium intake increases urine volume and reduces water intake.
Then this line blew me away:
“The resulting rhythmical Na+ storage and release reduced the predictive value of an accurately collected 24-hour urine sample to correctly estimate a 3-g/d difference in salt intake to only 50%”
Think of how many studies on sodium, hypertension and kidney stones have relied on the intuitive, simple principle of sodium in = sodium out . Burn it all down.
The authors then propose three interconnected hypotheses to explain long-term control of body fluids in humans.
Hypothesis 1: Increasing salt intake promotes accrual of endogenous water
Hypothesis 2: Dietary salt modulates endogenous infradian- rhythmical control of osmolyte and water accrual and release.
Hypothesis 3: High salt intake induces glucocorticoid-driven metabolic water production.
This is an important study that reveals truths of the human body that show sodium and water handling is more complex than the models we have used for over half a century. We have built entire kingdoms on the assumption that sodium excretion is equal to dietary sodium intake in neat 24-hour intervals. Like any research, this needs to be replicate. But getting people to sign up for 100-day metabolic studies is not going to be forthcoming. It is amazing that it took 10 cosmonauts sealed into a simulated space station for 500 days to topple those ideas.
If these data are accurate
We need to stop relying on the 24-hour urine as unquestioned truth
and
We need to start thinking of glucocorticoid as a major influencer in sodium and water metabolism
It's a brave new world.














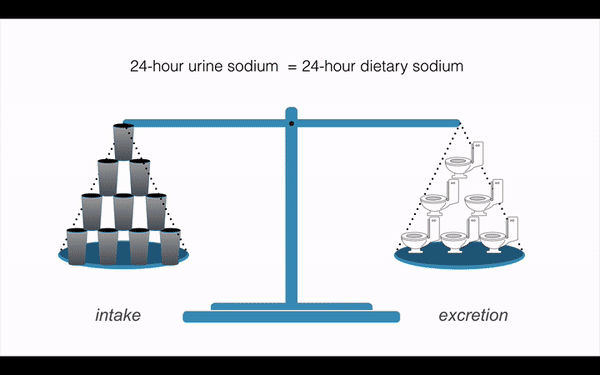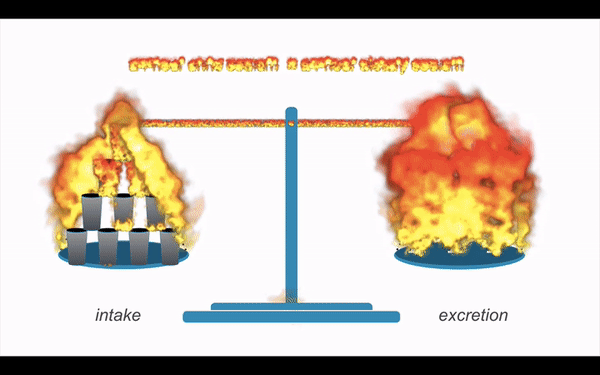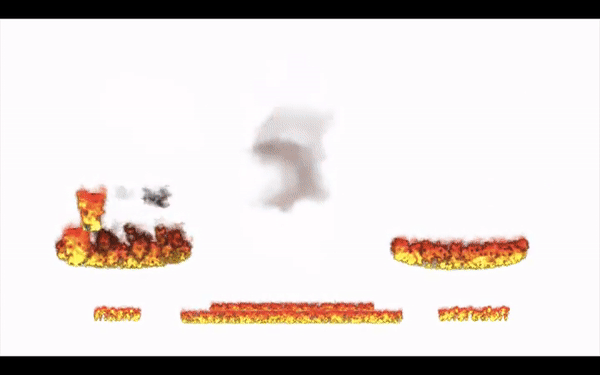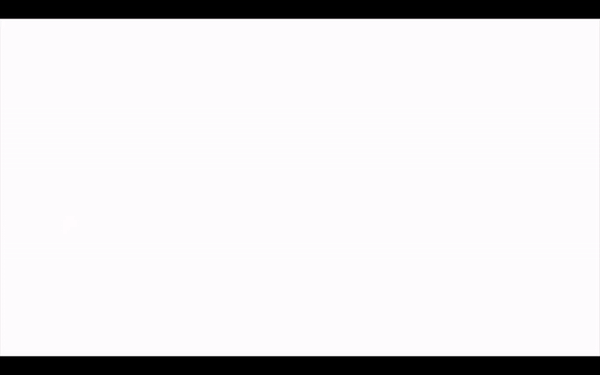
Follow us as we burn down everything you love about renal physiology. Oh, and learn how cortisol regulates urine volume.