#NephJC Chat
Tuesday Jul, 19, 2021 at 9 pm Eastern
Wednesday Jul, 20, 2021 at 9 pm Indian Standard Time
Wednesday Jul, 20, 2021 at 9 pm GMT (or BST)
JAMA. 2021 May 18;325(19):1946-1954. doi: 10.1001/jama.2021.4807.
Effect of Treating Hyperphosphatemia With Lanthanum Carbonate vs Calcium Carbonate on Cardiovascular Events in Patients With Chronic Kidney Disease Undergoing Hemodialysis: The LANDMARK Randomized Clinical Trial
Hiroaki Ogata, Masafumi Fukagawa, Hideki Hirakata, Tatsuo Kagimura, Masanori Fukushima, Tadao Akizawa, LANDMARK Investigators and Committees
PMID: 34003226
Introduction
Hyperphosphatemia is associated with increased cardiovascular mortality and all-cause mortality in patients with ESRD (Block et al., JASN 2004; Ganesh, et al. JASN, 2001) and CKD (McGovern et al. PLoS One, 2013; Palmer et al. JAMA, 2011) (see forest plot below). Based on a systematic review by Palmer, et. al., (Palmer et al, JAMA 2011) every 1 mg/dl increase in serum phosphate level is associated with an 18% higher mortality.
Serum phosphate and all-cause and cardiovascular mortality (Palmer et al., 2011)
In a healthy state, the skeletal system acts as a reservoir for phosphate (see figure below from Hruska et al., 2008).
Phosphorus balance in normal physiology (Hruska et al., 2008)
However, in CKD, complex alterations in calcium and phosphorus handling results in demineralization of the bones while the vascular system takes up the role of phosphorus reservoir (see figure below from Hruska et al., 2008). Increasing levels of phosphate in the vasculature is associated with vascular calcification (Hruska et al., 2008). Biology and epidemiological studies support a role for phosphate being a poison. Something we should try to lower to improve outcomes, right?
Phosphorus homeostasis in CKD/ESRD (Hruska et al., 2008)
Since we think hyperphosphatemia in CKD has to be bad, we manage this by moderating dietary phosphate intake and by using phosphate binders. Notably, none of the interventions that lower serum phosphate levels have been consistently shown to improve the clinical outcomes in clinical trials, especially when compared to placebo. This systematic review and network analysis of 77 trials (Palmer et al, AJKD 2011) found no increase in all-cause mortality comparing different types of phosphate binders to placebo. These trials, however, had short follow up time (~1-3 months) and there was insufficient data to determine CV risk. Why should there be such a discrepancy between the biological plausibility, the epidemiological data, and clinical trials? Many things, primarily including confounding since high phosphate often associates with poor adherence, lower dialysis adequacy, and other factors that might be the real culprit in this relationship. Whether lowering phosphate at all, and to what extent, is beneficial in patients with ESRD is a question that will be answered in a few years, when the PHOSPHATE and HiLO trials conclude. Check out the NephTrials and AJKD Blog posts for a discussion of these ongoing trials.
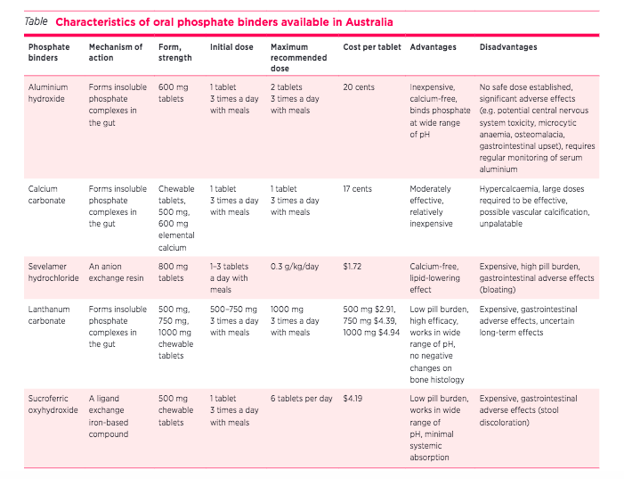
A separate question from whether and to what extent one should lower phosphate is how to lower phosphate. There are many different types of phosphate binders that are available. These fall under three categories: calcium-containing phosphate binders (CaCO3), aluminum-containing phosphate binders (AlOH3) and the newer non-calcium based phosphate binders (sevelamer hydrochloride, lanthanum carbonate and sucroferric oxyhydroxide). Their advantages and disadvantages are summarized below (from Chan et al., 2017, infographic by Dr. Missy Hanna) Given the risk of calcium-based phosphate binders causing hypercalcemia, there are concerns with the risk of accelerating vascular calcification (Chertow et al., 2002 Ohtake et al., 2013) again from observational studies and not supported from trials (see Hiremath et al, Lancet 2014). Despite this, as is common in the nephrology guideline world, KDIGO (2017 MBD Guideline update) states that “In adult patients with CKD G3a–G5D receiving phosphate-lowering treatment, we suggest restricting the dose of calcium-based phosphate binders” (Guideline 4.1.6. Graded 2B).
Different types of phosphate binders available (Chan et al., 2017)
In a 2018 systematic review of 30 RCTs or quasi RCTs comparing lanthanum vs calcium-based binders found no difference in all cause mortality - RR 0.76 (95%CI 0.18 to 3.11), low certainty evidence, however none of the studies looked at CV risk (Ruospo et al., 2018). Lanthanum is a potent phosphate lowering agent (like aluminum, and unlike sevelamer) but there has been some concern of long term accumulation of Lanthanum (Drueke Semin Dial 2007), again with memories of aluminum which the renal community used for decades before its link to low turnover bone disease and dementia became clear (though arguably this may be more from water than binders, see Mudge et al BMC Nephrol 2011). The other issue is that of establishing its superiority over calcium based phosphate binders - which are cheaper. The LANDMARK trial aimed to compare the risk of CV mortality and events in HD patients with at least 1 risk factor of vascular calcification who were randomized to calcium carbonate or lanthanum carbonate.
The Study
Design
This trial was an open-labelled, end-point blinded, randomized controlled trial in 273 Japanese hemodialysis centers
Randomly assigned 1:1 to the two groups using the minimization method with age (≤65 or >65), biological sex, diabetes and study site as qualifiers.
Minimum 3 year observation period
Intervention
Group 1: Lanthanum carbonate at an initial dose of 750 mg/day (divided into 3 doses) after meals or previously prescribed dose. Titrated to a maximum of 2250 mg/day to achieve a serum phosphate level between 3.5mg/dL and 6 mg/dL (1.13 - 1.94 mmol/L) . If the target range was not achieved, sevelamer hydrochloride or a different calcium-free phosphate binder could be added. Calcium carbonate could not be used in this group.
Group 2: Calcium carbonate at a dose of 3000 mg/day (divided into 3 doses) after meals or as previously prescribed. Titrated as appropriate to achieve a serum phosphate level between 3.5 mg/dL and 6 mg/dL (1.13 - 1.94 mmol/L). If target range was not achieved, sevelamer hydrochloride or a different calcium - free phosphate binder could be added. Lanthanum carbonate was not allowed in this group.
Both drugs were administered within 14 days of enrolment and continued for 3 years after last patient enrolment.
Study Population
Patients on hemodialysis for 3 months of longer receiving phosphate binder treatment
At least 1 of the following risk factor for vascular calcification:
Age ≥ 65
Postmenopausal
T2DM
Intact PTH level of <240 pg/mL
Anticipated life expectancy of ≥ 1 year
Key exclusion criteria:
Currently undergoing PD
Contraindications of lanthanum carbonate or calcium carbonate (ischemic heart disease, stroke within 6 months of study enrollment, NYHA class III-IV heart failure, arrhythmia requiring treatment, severe liver dysfunction, severe malnourishment, or malignancy within 5 years prior to enrollment)
Pregnant, possibly pregnant or lactating, or anticipating pregnancy during the study period
Ineligible per investigator’s judgement.
Measurements
Followed the Japanese Society for Dialysis Therapy Clinical Practice Guidelines for Management of CKD-MBD.
Target range for 1st day of dialysis each week for serum phosphate was 3.5 - 6.0 mg/dL (1.13 - 1.94 mmol/L), corrected calcium was 8.4 - 10.0 mg/dL (2.1 - 2.5 mmol/L) and iPTH was 60 - 240 pg/mL (6.36 -25.5 pmol/L)
Outcomes
Primary outcome: composite of CV events
Death due to CV event (MI or stroke) and cardiac death
Nonfatal MI
Nonfatal stroke including TIA
Unstable angina
Hospitalization for heart failure
Hospitalization for ventricular arrhythmia
Secondary outcomes:
Overall survival
Secondary hyperparathyroidism-free survival
Hip fracture-free survival
Adverse events
collected for 30 days after completion of treatment
Post hoc addition of CV death
Statistical analysis
Initial planned sample size of 3000 assuming a CV event rate of 7.8% (nationwide Japanese survey), with the expected reduction of events by 1.5% in the lanthanum carbonate group. Therefore, anticipated HR of 0.8 with a two sided type 1 error rate of 5%, power of 80% and an expected attrition rate of 10%.
This sample size was not achieved - power was decreased to 0.68 and a sample size of 2296 was used.
Intention to treat analysis with per protocol sensitivity analysis
Funding
Funded by Bayer Yakuhin Ltd.
Bayer Yakuhin Ltd. had no role in the design of the study, data collection, management, analysis or interpretation, manuscript preparation, review or approval.
Funder was kept informed about study progress
Results
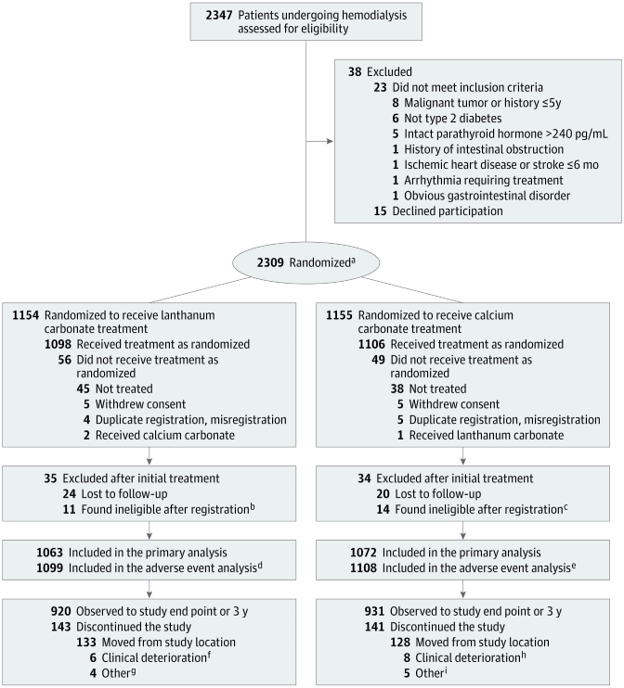
A total of 2,347 participants were screened during the enrollment period from (November 2011 to July 2014), 2,309 participants were randomized to the two groups and the participants were followed until Jun 26, 2018. Of these, 2,135 participants were included in the final analyses (lanthanum carbonate n=1063 and calcium carbonate n=1072). In the lanthanum carbonate group 4.9% (n=56) did not receive treatment and 3.2% (n=49) in the calcium carbonate group did not receive treatment. The reasons for not receiving treatment were similar in both groups. Follow up was not completed for 13.5% in the lanthanum carbonate group versus 13.2% in the calcium carbonate group with the reasons being similar in both groups (Figure 1). Follow up was similar in both groups median (IQR) (3.16 yrs (2.41 - 3.89) - lanthanum carbonate group vs 3.16 yrs (2.55 - 3.85)- calcium carbonate group).
Figure 1. Participant selection and flow
Baseline characteristics
Baseline characteristics are shown below in Table 1. Median age of the participants in this trial was 69 years old and 40% of the participants were female. The median dialysis vintage was just under 5 years, ~ 125 were current smokers, and ~ 55% had diabetes. However, mean weight was about 56 kg, and BMI only 22 (quite different from most European/American populations). Previous history of cardiovascular disease was also uncommon (see below). About 70% were on calcium carbonate, and 30% on lanthanum, with about 20% on sevelamer at baseline.
Table 1 Baseline characteristics
At the beginning of the study the median daily dose of lanthanum carbonate was 750 mg (IQR, 750-1500 mg) and calcium carbonate was 1500 mg (IQR, 1500-3000 mg). During the study period while the lanthanum carbonate daily dose increased to 1500 mg/d, the daily dose of calcium carbonate remained unchanged. Use of sevelamer in the lanthanum carbonate group remained the same through the study period ~18%, the use of sevelamer increased from 20.2% to 27.2%. In contrast, in the lanthanum carbonate group the use of active Vitamin D increased compared to baseline, while it remained stable in the calcium carbonate group. No difference was found in cinacalcet use in the two groups (e figure 2).
eFigure 2. Concomitant use of sevelamer hydrochloride, active Vit D and cinacalcet
CKD MBD parameters
During the course of treatment, serum phosphate levels decreased significantly more in the calcium carbonate group compared to the lanthanum carbonate group (p<0.001). In contrast, changes in serum calcium levels were higher in the calcium carbonate group in the lanthanum carbonate group compared to baseline (p<0.001). Calcium x phosphate product increased overall in both groups but was not significantly different. (e figure 5)
eFigure 5. Changes in serum phosphate, corrected calcium and calcium x phosphate product from baseline.
However if one looks at mean levels of phosphate, calcium and calcium x phosphate product, they were not quite different between groups (see eFigure 3 below). .
eFigure 3. Median serum phosphate, corrected calcium levels, and calcium x phosphate products over time.
Primary endpoint
There was no significant difference in the composite CV endpoint between the two groups. The endpoint occurred in 147 of 1063 participants in the lanthanum carbonate versus 134 of 1072 participants in the calcium carbonate group (absolute difference 0.50 events per 100 person-years [95% CI -0.57 to 1.56], see Figure 2).The adjusted HR was 1.11 [95% CI, 0.88 to 1.41]. Per-protocol analysis revealed similar results.
Figure 2. Primary Composite and All-Cause Mortality Outcomes
Stratified subgroup analysis for age, sex, CKD BMD parameters, CVD risk factors, dialysis adequacy or ACE-i/statin/ASA use showed no significant difference in event rates (see eFigure 7).
eFigure 7. Prescpecified subgroup analyses- primary composite outcomes
Secondary endpoint
There was no difference in all cause mortality between the two groups. A total of 159 deaths occurred in the lanthanum group 4.96 deaths per 100 person-years [95% CI 4.33 to 5.65] vs 148 in the calcium carbonate group : 4.53 deaths per 100 person-years [95% CI 3.93 to 5.19] with a HR of 1.10 [95% CI, 0.88 to 1.37] (see table 2).
CV mortality was higher in the lanthanum carbonate group (n=58) compared to calcium carbonate (n=39) with a HR of 1.51 [95% CI: 1.01-2.27] (p=0.045). Risk of hip fracture was not significantly different between the two groups. (Table 2).
Secondary hyperparathyroidism free survival- defined as the length of time from the date of enrollment until the date of onset of secondary hyperparathyroidism- was higher in the lanthanum carbonate group (n=105) compared to calcium carbonate (n=68) with a HR of 1.62 [ 95% CI 1.19- 2.20] (p=0.002).
Table 2. Hazard ratios for primary composite and secondary outcomes
Adverse events
In the lanthanum carbonate group, 282 (25.7%) adverse events were reported compared to 259 (23.4%) in the calcium carbonate group. The most common adverse events in both groups were GI disturbances (63.8% in the lanthanum carbonate group vs 48.6% in the calcium carbonate group). As mentioned previously, hyperphosphatemia was more common in the lanthanum carbonate group with 30.1% at the end of the study period compared to 17.4% in the calcium carbonate group. In contrast, hypercalcemia was less common in the lanthanum carbonate group with 5.7% at the end of the study period compared to 6.7% in the calcium carbonate group (eTable 5).
eTable 5. Rate of hyperphosphatemia, hypophosphatemia, hypercalcemia and hypocalcemia
Discussion
The LANDMARK trial is a multicenter, open-labelled, blinded endpoint RCT which showed no significant difference between lanthanum carbonate and calcium carbonate in the risk of composite CV endpoint and all-cause mortality in patients on hemodialysis with 1 vascular risk factor. The lanthanum carbonate group had a higher risk of CV death on post-hoc analyses. Even for all other clinical outcomes, the point estimate was (non-significantly) worse for lanthanum.
Strengths
Study design and population: This is a well designed RCT (and the largest to date) comparing the risk of CV events, mortality and all cause mortality with calcium-based vs calcium-free phosphate binders. Although the participants were not blinded, the end-point evaluation was blinded. The follow up period of a minimum of 3 years ensured sufficient time for events to occur.
Minimization method was used for randomization with the particular inclusion of study site to allow for site specific differences in ESRD care, and age, sex and diabetes to account for CV risk factors.
The attrition rate, while higher than anticipated, was equal in both groups and for similar reasons.
Limitations
This study was not double-blinded.Underlying bias due to physician comfort with using one phosphate binder over another and the concurrent diet counseling based on types of phosphate binder cannot be accounted for.
This study may be underpowered to assess CV risk as the rates of CV events were much lower in this study population than anticipated and due to the study not meeting the original target for recruitment.
Patient adherence to medication in each group was not reported. Additionally, dialysis frequency, dialysis duration and adherence to dialysis was not reported in each group.
It is unclear if the higher adjunctive use of sevelamer in the calcium carbonate group altered CV risk thereby biasing the result towards the null.
Similarly, higher use of activated vitamin D in the lanthanum carbonate group may play a role in CV risk. However subgroup analysis based on VDRA (though at baseline) did not show any overt difference.
Given this study was conducted in Japan where dietary practices and CV screening practices result in lower CV events and risk, it is unclear whether these results can be generalizable to other populations. Additionally, this study may not be applicable to PD patients and nocturnal dialysis patients.
While CV mortality was higher in the lanthanum carbonate group, this analysis was not prespecified, the possibility of an incidental significant analysis in the context of multiple comparisons should be taken into consideration.
Conclusion
Hyperphosphatemia can be managed with calcium-based or calcium-free phosphate binders. The current KDIGO guidelines recommend (2B) limiting the use of calcium based binders due to the risk of hypercalcemia and potentially accelerating vascular calcification (KDIGO 2017 update MBD guidelines). This trial shows clearly that the KDIGO guideline is wrong. We don’t know what may be the mechanism of higher CV mortality with Lanthanum. Could it be the higher sevelamer use in the calcium group (though sevelamer has not shown that effect in any RCT, eg. Suki et al Kidney Int 2007 and St Peter et al AJKD 2008)? Might it be that lanthanum itself is bad in some way? Or could it be the higher phosphate drop seen with the calcium group - though we expect that question to be answered more clearly by the ongoing HiLo (Edmonston et al AJKD 2021) and Phosphate trials. However, the myth of the inferiority of calcium carbonate approach has been debunked by this landmark trial. At least as far as lanthanum (in Japan) is concerned.
Summary prepared by
Sharanya Ramesh
Internal Medicine Resident
University of Toronto
Paresh Jadav
Department of Nephrology
Columbia University, NY
NSMC interns, Class of 2021