#NephJC Chat
Tuesday September 4, 9 pm Eastern
Wednesday September 5, 8 pm BST, 12 noon Pacific
J Am Soc Nephrol. 2018 Jun;29(6):1731-1740. doi: 10.1681/ASN.2017111213. Epub 2018 May 10.
Oral Antibiotic Exposure and Kidney Stone Disease.
Tasian GE, Jemielita T, Goldfarb DS, Copelovitch L, Gerber JS, Wu Q, Denburg MR.
PMID: 29748329 Full Text at JASN
Editorial by Nazzal and Blaser
Introduction
Kidney stones is a huge source of morbidity and healthcare utilization in the United States. Thirteen percent of men and 7% of women will experience a kidney stone over their lifetimes. Stones result in over 1.3 million annual emergency department visits. Antibiotic use is even more common. In 2015, there were 269 million antibiotic prescriptions in the United States, of which an estimated 30% were unnecessary.
The relationship between antibiotic use, the microbiome, and kidney stone formation is interesting. Kidney stone formers may have less microbial diversity in the gut microbiome compared with non-formers. Stone formers have been shown to have increased pro-inflammatory bacteria, including Megamonas, Phascolarctobacterium, Escherichia-Shigella, and Sutterella.
As seen in NephMadness 2014, Oxalobacter formigenes plays a role in stone formation. O formigenes consumes oxalate in the GI tract, decreasing oxalate absorption and urinary excretion. Antibiotics decrease O. formigenes, which may increase oxaluria and predispose to stone formation. O. formigenes is susceptible to many outpatient antibiotics including antibiotic courses for H. pylori. Stone formers may have less gut O. formigenes, but the relationship between this and urinary oxalate excretion is not entirely clear, suggesting other components of the microbiome are likely involved.
Given these proposed biological mechanisms, the authors embarked on a large, observational study to assess the relationship between antibiotics use and kidney stone formation.
The Study
Methods
The study used a case-control design nested within the The Health Improvement Network (THIN) cohort in the United Kingdom, which has data on 13.8 million patients in 641 general practitioner (GP) practices. Kidney stone formers were determined by diagnosis codes. Patients with kidney stones were matched 1:10 with controls by age, sex, and GP practice and compared using conditional logistic regression models.
The primary exposure was an outpatient oral antibiotic prescribed 3–12 months before the kidney stone diagnosis date. Because GPs in the UK serve as gatekeepers and work in an integrated system, the investigators had highly accurate prescribing information.
Three models were used to adjust their analyses. All models were adjusted for disease, UTI, health care encounter rate, and prescriptions for PPIs, statins, and diuretics, due to their association with kidney stones. The second model additionally adjusted for antibiotic prescriptions other than the primary exposure within 3–12 months. The third model was adjusted for antibiotic prescriptions according to time window prescribed and other indicator variables. They also looked at factors related to utilization, including number of doctor and ED visits, as well as imaging studies.
Results
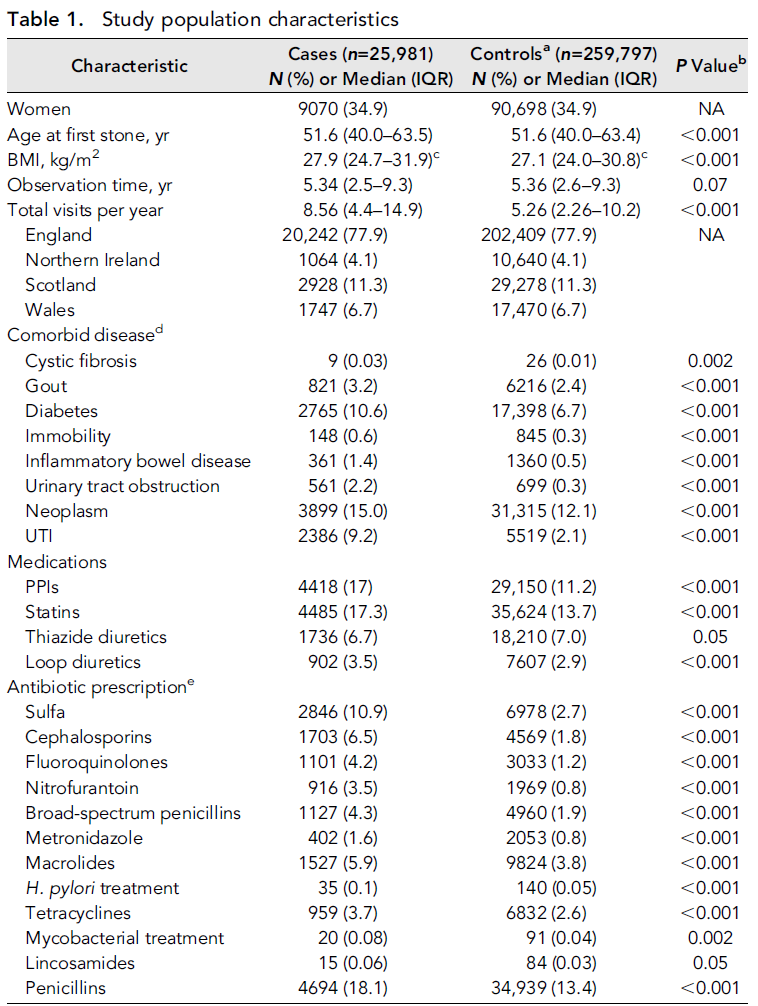
25,981 patients with kidney stones and 259,797 controls were included in the study sample. Median follow up was 5.4 years (Table 1). Patients were mostly male (65.1%) and located in England (77.9%). As expected, patients with stones were quite different than those who did not have stones: eg rates of diabetes, neoplasm, and UTI were higher in patients with kidney stones. Rates of PPI and statin use were also higher in kidney stone formers.
Table 1 from Tasian et al, JASN 2018
Sulfa antibiotics were associated with the highest odds of kidney stone formation in fully adjusted analyses (OR 2.33; 95% CI 2.19 to 2.48), followed by cephalosporins (OR 1.88; 95% CI 1.75 to 2.01) and fluoroquinolones (OR 1.67; 95% CI 1.54 to 1.81).
Table 2 from Tasian et al. For more on Bonferroni, check out Manasi's post from March
The odds of kidney stone formation based on antibiotic class were significantly higher at younger ages (Figure 1).
Figure 1, from Tasian et al. JASN 2018. Odds of kidney stone disease were greatest for antibiotic exposures at younger ages.
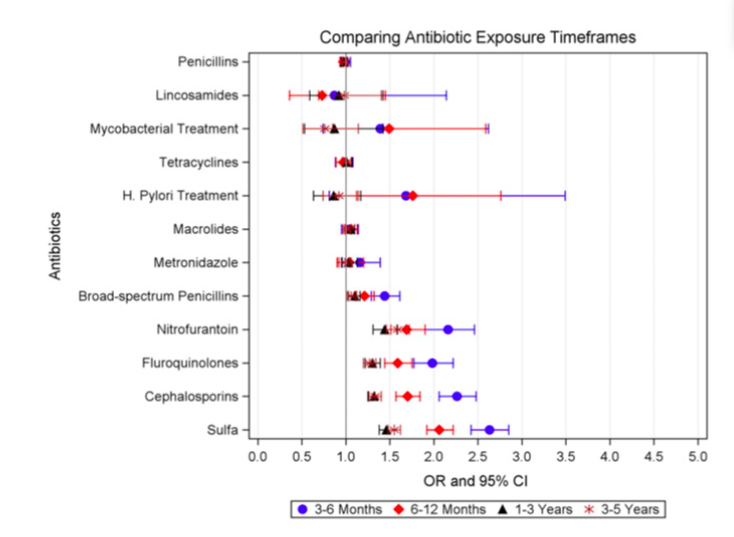
The Odds of kidney stones were also higher if antibiotic exposure was more recent (Figure 2, and table 3 from paper).
Figure 2 from Tasian et al, JASN 2018. Odds of kidney stone disease were greatest for more recent antibiotic exposures.
Sensitivity analyses which excluded patients with UTI, excluded short (<5 days) or long (>6 month) treatment durations, adjusted for obesity, excluded nonqualifying stone codes, and adjusted for diagnostic imaging all produced similar results as the primary analysis (table 4 in paper).
Discussion
The authors found that outpatient prescriptions of several antibiotic classes—sulfas, cephalosporins, fluoroquinolones, nitrofurantoin/methenamine, and broad-spectrum penicillins—were associated with higher odds of kidney stone formation at 3-12 months.
The study impressively used a large number of subjects and had very accurate prescribing data to ascertain the exposure. Matching subjects to controls based on GP practice, in addition to age and sex, decreased the impact of unmeasured confounders in the analysis. While patients with kidney stones had increased rates of UTIs and antibiotic prescriptions, and UTIs may be related to the presence of stones, the results remained when adjusting for these factors, as well as multiple other sensitivity analyses. Biological plausibility and a time-response relationship further support the findings (Figure 2). The authors accounted for increased rates of diagnosis of incidental kidney stones by adjusting for imaging utilization. They acknowledge that there may be unmeasured confounders, such as diet, fluid intake, or comorbidities, which may be different between the two groups.
Interestingly, previous data have shown that strains of the gut bacterium Oxalobacter formigenes, which may impact oxaluria, were not susceptible to several penicillins and cephalosporins. This suggests that other gut microbes which compose the “oxalobiome,” such as Bifidobacterium, Lactobacillus, and Escherichia coli, or alternate mechanisms entirely are likely contributing (as the editorialists suggest).
The results generate more hypotheses with regards to biological mechanisms than where we began. An open question is whether antibiotic exposure affects kidney stone formation for reasons independent of gut microbiome. Do antibiotics change urinary pH, citrate, phosphate, calcium, or other electrolytes affecting kidney stone formation? Measuring the concurrent effects of antibiotics on the gut microbiome and urine parameters would be particularly powerful.
Here we find another argument for reducing inappropriate use of antibiotics, and a potentially modifiable risk factor that could decrease rates of kidney stones. That’s a win-win-win for our gut, our kidneys, and the healthcare system!
Summary by Sri Lekha Tummalapalli,
Nephrology fellow, UCSF
NSMC Intern, class of 2018
Banner Image from Wellcome group collection, credit: Sergio Bertazzo, Imperial College London; Dominique Bazin, UPMC; Chantal Jouanneau, INSERM. Used under CC BY 4.0.