Is 120 the new 140?
A Randomized Trial of Intensive versus Standard Blood-Pressure Control.
SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT.
Collaborators (844)
November 9, 2015 DOI: 10.1056/NEJMoa1511939
PMID: 26551272
#NephJC tweetchat
Tuesday Nov 10 9 pm EDT
Wednesday Nov 11 8 pm GMT and 12 noon PST
The Landmark SPRINT trial results were finally revealed in all their glory on Nov 9th at 2 pm EDT at the AHA meeting in Orlando. Before we discuss the results, here are some of the previous publications from the SPRINT research group:
The protocol is available here (free PMC link)
Protocol for BP management from Sprint Trial page (PDF link) and lots of other details available at official SPRINT trial page
Pre-SPRINT systematic review of intensive BP control in the Lancet, by Vlado Perkovic et al
Pre Chat Summary of SPRINT Methods and Results
The SPRINT trial, funded by the NHLBI arm of the NIH (and co-sponsored by the NIDDK, NIMDS and NIA), was based on a working group report (PDF link) from 2007, which identified the question of targeting low blood pressure (i.e. 120 mm Hg) in non-diabetics (since ACCORD was ongoing in Diabetics then) as a priority.
Methods
Overall, it took a whopping 102 centers (grouped under 5 clinical center networks) to enroll the patients into this trial.
Inclusion Criteria:
At least 50 years old
Systolic blood pressure
SBP: 130 – 180 mm Hg on 0 or 1 medication
SBP: 130 – 170 mm Hg on up to 2 medications
SBP: 130 – 160 mm Hg on up to 3 medications
SBP: 130 – 150 mm Hg on up to 4 medications
There are no diastolic blood pressure (DBP) inclusion criteria
Risk (one or more of the following):
a) Presence of clinical or subclinical cardiovascular disease other than stroke (based on detailed definitions)
b) CKD, defined as MDRD eGFR 20 – 59 ml/min/1.73m2 within the past 6 months.
c) Framingham Risk Score for 10-year CVD risk ≥ 15%
d) Age ≥ 75 years.
Selected Exclusion Criteria
An indication for a specific BP lowering medication
Known secondary cause of hypertension that causes concern regarding safety of the protocol.
One minute standing SBP < 110 mm Hg. Not applicable if unable to stand due to wheelchair use
Proteinuria (> 1g/day or albuminuria >600 mg/day or equivalent PCR/ACR or 2+ on dipstick)
Arm circumference too large or small to allow accurate blood pressure measurement with available devices
Diabetes
History of stroke (not CE or stunting)
Diagnosis of polycystic kidney disease
Glomerulonephritis treated with or likely to be treated with immunosuppressive therapy
eGFR < 20 ml/min /1.73m2 or end-stage renal disease (ESRD)
Cardiovascular event or procedure (as defined above as clinical CVD for study entry)
A medical condition likely to limit survival to less than 3 years,
Any factors judged by the clinic team to be likely to limit adherence to interventions.
Residence in a nursing home. Persons residing in an assisted living or retirement community are eligible if they meet the other criteria.
Any organ transplant
Intervention
This was a randomized, controlled, open label trial. Eligible participants were assigned to a systolic blood-pressure target of either less than 140 mm Hg (the standard-treatment group) or less than 120 mm Hg (the intensive-treatment group). The study investigators were not blinded - but the outcome adjudicators were blinded. BP was measured quite meticulously with automated oscillometric BP devices (which discard the first reading, and take 3-5 consecutive readings and give an average) which approximate true resting blood pressure reasonably. Patients were seen quite frequently (and decreasing/stopping medications if BP was too low, especially in the standard arm was a notable feature). The algorithms and formulary can be seen from the supplementary figures from the appendix:
Figure S1
Figure S2
Table S1
Results
As planned, 9361 participants were enrolled. Details can be seen in figure 1 and table 1. Of note, ~2600 patients had CKD (eGFR 20-59) making it one of the largest hypertension trials in this population.
Figure 1
Table 1
There was a nice separation of the systolic and diastolic blood pressures (seen in figure 2 and Supp figure S4).
Outcomes
With respect to the primary outcome (a kind of MACE outcome, including heart failure), there was a nice separation at one year, with an overall HR of 0.75 (favouring intensive treatment) and an NNT of ~ 60 over 3.7 years. For all-cause mortality, this difference is noticeable at about 2 years, with a HR of 0.73 (and an NNT ~ 90 over the 3.7 years).
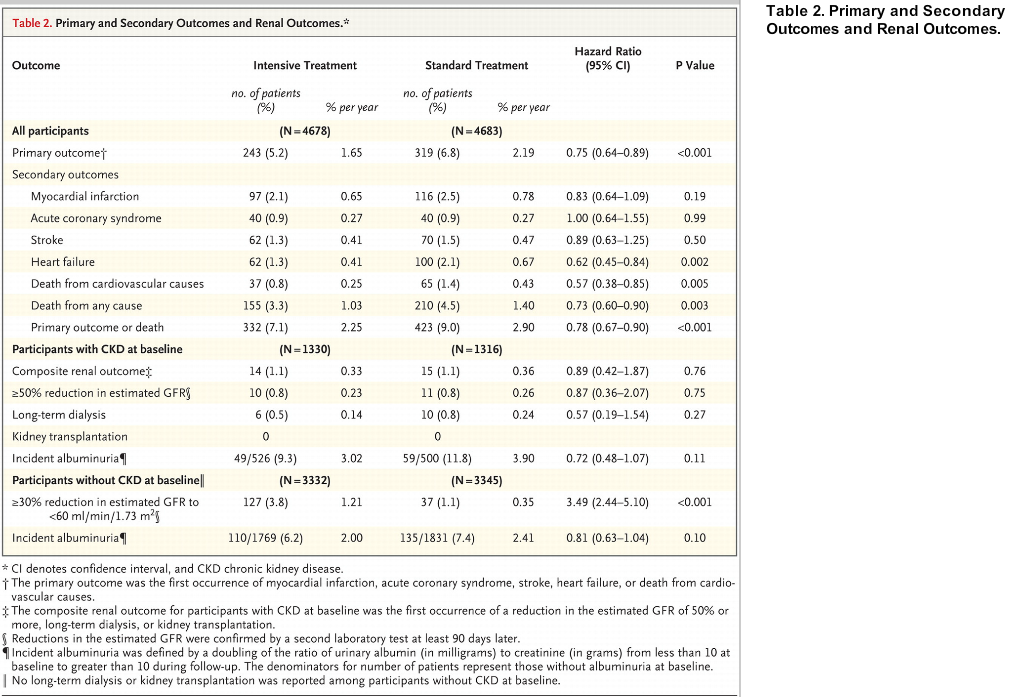
The details of the primary outcome are available in table 2 (notable being the decrease in eGFR seen in the intensive arm in the subgroup with GFR > 60)
Table 2 from SPRINT
Notably, the intensive blood pressure arm resulted in no increase in overall adverse events (though note the increase in AKI)
Table 3
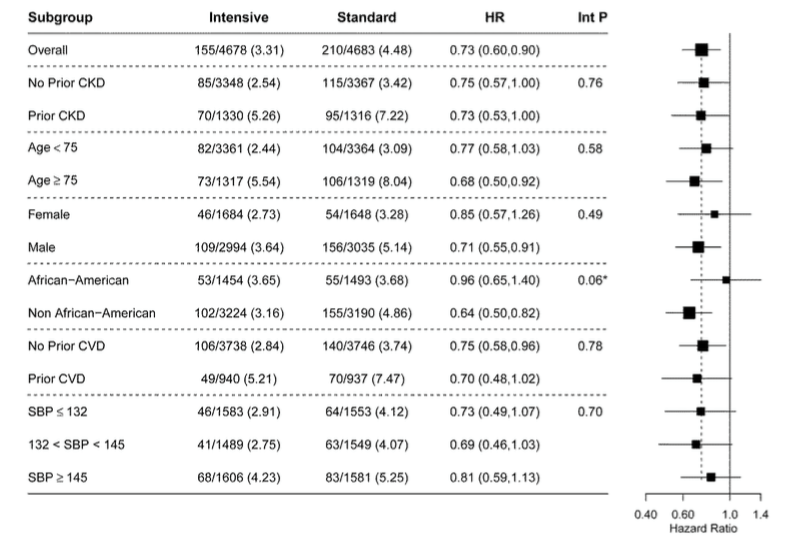
There were a lot of pre-specified subgroups, and none of them displayed any heterogeneity (see figure 4 for primary outcome, and figure S5 for mortality - shown below)
Discussion
Apart from the discussion in the article itself, there were many simultaneous publications:
In NEJM
A nicely written editorial (with some additional meta-analysis) from Vlado Perkovic (who also has done a Youtube interview) and Anthony Rodgers
Another perspective from Aram Chobanian
An editorial from the NEJM editors, explaining their decision making
A clinical case discussion vignette piece
A quick take video
A podcast interview with author Dr Whelton
In the Hypertension
3 editorials (PDF available at this link)
New York Times
A favourable article by Gina Kolata
Another perspective from Harlan Krumholz
Medscape
An overview from Deborah Brauser
A typically thoughtful piece from John Mandrola
On the ACC website
Multiple quick-read posts, by George Bakris (who says, "The findings related to all-cause mortality are a mystery to me and have no plausible explanation"!), Susan Steigerwalt and Roger Brook and Tim Eagle
An analysis by Adam Bress et al using NHANES in JACC, suggesting 20% US adults with hypertension (~ 16 million overall) would fit SPRINT criteria
JASN
A commentary by Glenn Chertow et al (who were actually involved in SPRINT)
Wikidoc C.Michael Gibson
Interview with Author: Methods and Results
A critical take from Alan Cassels
#NephJC tweetchat
Tuesday Nov 10 9 pm EDT
Wednesday Nov 11 8 pm GMT and 12 noon PST
Summary prepared by Swapnil Hiremath