Infographic by Namrata Parikh @NamrataYParikh
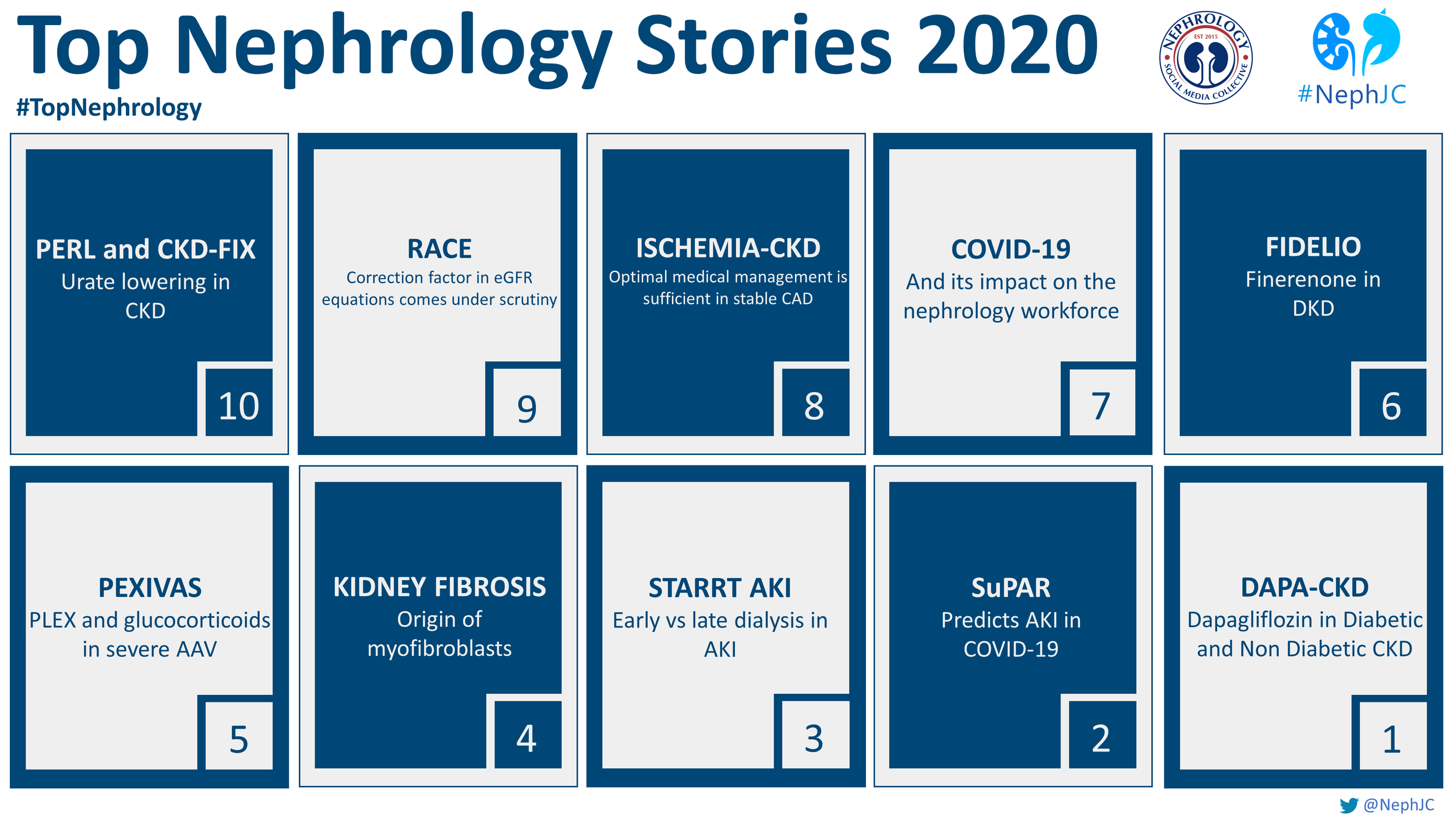
10: PERL and CKD-FIX- Urate lowering in CKD
2020 blessed us with not one but two clinical trials published in the NEJM aiming to answer the age old question of whether uric acid is simply a marker of CKD or contributes to the progression of CKD. First, the PERL study enrolled adult patients with type 1 diabetes, a serum urate level of 4.5 or greater, and eGFR of 40-99.9 ml/min/1.73m2 and randomized them to treatment with allopurinol or placebo for 3 years. After a 2 month washout, the primary outcome of baseline adjusted iohexol GFR was essentially the same in both groups. The second study, CKD-FIX, enrolled adults with CKD stage 3 or 4 with either an albumin/creatinine ratio of ≥265mg/g OR an eGFR decline of at least 3ml/min/1.73m2/year and randomized them to receive allopurinol or placebo. There was no difference between groups in the primary outcome of change in eGFR over 2 years. There was a significant decrease in serum urate levels in both studies, however no changes in kidney outcomes. So should we throw our allopurinol out the window and stop checking uric acid levels? Not everyone thinks so. Maybe we’ll see urate back in the top ten sometime in the future.
9: Race correction factor in eGFR equations comes under scrutiny
The race coefficient, used in eGFR equations, came under intense scrutiny in 2020 (listen to the Freely Filtered Podcast on this with guests Dr. Deidra Crews, Dr. Poyan Mehr, and Carina Seah). This issue highlights the real problem that needs to be tackled head on- institutional racism, health inequities, and the disproportionate number of people who are Black or African American who are on dialysis. It is striking. Kidney failure prevalence is nearly 4 times greater in people who are Black or African American and comprise 33% of the US dialysis population but 13% of the general US population. We have so much work to do. However, it needs to be pointed out that the incorporation of race in estimating equations for GFR were already under fire well before 2020. Several in the nephrology community were already questioning this (see this NephJC chat in 2019). The inclusion of the race coefficient dates back to the MDRD eGFR equation in 1999, as race was included as a demographic variable in the MDRD study (how race was ascertained in this study is now being questioned). The CKD EPI equation also uses a race coefficient (Black or other). The crux of this issue is that race is not a biologic variable but a sociopolitical construct that does not predict biology. Furthermore, there is inconsistent practice on how race is ascertained and issues surrounding mixed race and how people self identify as a particular race. However, it must also be noted that controversy exists surrounding this issue. Several institutions have already removed the race coefficient. Because of these issues, ASN and NKF convened a national joint task force to reassess the inclusion of race in diagnosing kidney disease with results expected in early 2021.
8: ISCHEMIA-CKD: optimal medical management is sufficient in stable CAD
One of the rallying cries of people concerned about Renalism is that patients with advanced CKD are denied life saving coronary angiograms because of a misguided fear of contrast nephropathy, but what if the coronary angiograms were not life saving? This question was put to the test in a landmark randomized controlled trial, Ischemia-CKD. Only patients with moderate to severe angina with a positive stress test and an eGFR < 30 ml/min or dialysis. That is not a typo, while “eGFR < 30 or dialysis” is part of the exclusion criteria of every major cardiovascular trial ever, here it is an inclusion criteria. Patients were then randomized to an invasive strategy that started with a cardiac angiogram followed by interventions as indicated or a conservative strategy otherwise known as optimal medical management. What happened? Well, those cath cowboys can hang up their lead, because there was no difference in the primary (composite of death or nonfatal myocardial infarction) or secondary outcomes (composite of death, nonfatal myocardial infarction, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest). But an interventional strategy must’ve improved angina? Nope. Didn’t even improve Seattle Angina Scores.
How does renalism die? Under the boot of an RCT showing that coronary angiograms were never the cat’s pajamas anyway. (See The ICD2 Trial for another example of renalism falling to the power of an RCT)
7: COVID-19 and its impact on the nephrology workforce
N-95s. Surge planning. Fear. Masks. AKI. Lockdowns. Telemedicine. Creating your own CRRT fluid. PPE shortages. Loneliness. Acute start PD. Resolve. COVID19 literature boom. (but not for everyone). Virtual conferences. Homeschooling. Hand sanitizer. Anger. Salary cuts. Deaths. Facetime goodbyes. COVAN. Government incompetence. Zoom. Teamwork. Virtual connections. Vaccines. Hope?
2020 was a year like no other for nephrology. We will never be the same.
6: FIDELIO- Finerenone in DKD
Ever since the discovery of angiotensin receptor blockers nephrologists have dreamed of dual RAAS blockade. We wanted to believe it so much that when Nakao et al published COOPERATE in the Lancet we bought it for 6 years until it collapsed into a pile of fraud. We wanted to believe that adding an ARB to an ACEi would provide dramatic benefits to our patients by squashing proteinuria. But excess hyperkalemia and adverse renal outcomes in VALIANT, ONTARGET and VA-NEPHRON-D cured us of this. Alternative versions of this included ALTITUDE (Aliskiren, a direct renin inhibitor, was added to an ACEi or ARB. Lancet covered the renal outcomes of this abandoned trial) which revealed an unfortunate excess of nonfatal stroke, renal complications, hyperkalemia, and hypotension. But this year Bayer finally found a way to get dual therapy across the finish line and demonstrated a reduction in the progression of diabetic kidney disease as a reward for their efforts. FIDELIO was the largest diabetic kidney disease ever done. Fidelio was a multicenter, placebo-controlled, international, randomized trial of fineronone, a novel, non-steroidal aldosterone antagonist. This unusual drug gives up what was previously aldosterone antagonists’ strongest asset, an almost uncanny ability to treat resistant hypertension (see PATHWAY-2) while retaining aldosterone antagonists’ Achilles heel, the tendency to cause hyperkalemia (see AMBER). But despite this curious combination attributes, finerenone was able to reduce the progression of diabetic kidney disease in patients on maximum ACEi or ARB. Look for finerenone to try to get approval by the FDA in 2021 and add to the expanding options in the treatment of diabetic kidney.
5: PEXIVAS- Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis
PEXIVAS clinched the #5 spot in 2018 when it was presented, and is back at the same level in 2020, the year when it was published (Walsh et al, NEJM 2020), discussed and dissected (NephJC summary). ANCA vasculitis is rare, and severe ANCA vasculitis is even less common. How does one do a trial, especially one that is powered for hard clinical outcomes in this area? If we can bring the global community together, these trials are indeed possible, as is shown by the investigators in the case. The largest ever trial in ANCA vasculitis examined 2 different interventions: the safety of lower dose of steroids, and the efficacy of plasma exchange compared to standard of care. Sadly, infections are a major cause of death for patients with severe ANCA vasculitis, and situations where the cure may sometimes be worse than the cause of the disease. With more than 700 patients randomized, this trial was more than 5 times larger than the preceding MEPEX trial (Jayne et al, JASN 2007). The perceived negative outcome of the trial was that plasma exchange was not really superior to standard of care. This result is going to be sliced, diced, and analyzed in many different ways for years to come. There has already been a debate in the pages of NDT (Pro: Kronbichler et al, NDT 2020; CON: Specks et al, NDT 2020) , but the interested reader should also read this review (de Vriese et al, CJASN 2020) which examines all the excuses for still continuing plasma exchange, such as the lack of kidney biopsy results, or active disease, or severe pulmonary hemorrhage, and lays them to rest. At the most, there may be a possibility that it buys you a few months of dialysis freedom, which is transient and is washed away in the long-term follow-up. However, lost in the plasma exchange discussion is a far more important result:lower dose steroids are just as efficacious, and far more safe than the standard dose of steroids used until now. Infections are a common cause of mortality in ANCA vasculitis, to repeat ourselves. Steroids are nasty (see recent RFN blog post on this). Let us reduce immunosuppression and reduce the infections. Sometimes, by giving less, we are able to achieve more.
Swap
4: Origin of myofibroblasts in human kidney fibrosis
Fibrosis is the ultimate destination in all forms of kidney disease and represents irreversible organ failure. Thus, the need to understand the pathologic mechanism of fibrosis is urgent. Kuppe et al. reported in Nature a very interesting study using single cell RNA sequencing of mouse models of CKD and humans with and without CKD. They demonstrated that pericytes and fibroblasts are the major cellular source of kidney fibrosis. They identified nake cuticle homolog 2 (Nkd2) as a myofibroblast-specific target in human kidney fibrosis. This study helps to shed light on the cellular origins of kidney fibrosis and has the potential to develop novel strategies to target in the future. Check out the NephJC coverage here.
3: STARRT AKI- Early versus late dialysis in AKI
Lord Nelson once said, “I owe all my success in life to having been always a quarter of an hour before my time.” The nephrology community has been following that advice, albeit without the same success that Lord Nelson enjoyed in his battles with Napoleon. A decade ago, we found out that starting chronic dialysis in patients on the basis of a low GFR was not really the right thing to do, when compared to waiting until symptoms develop at an even lower GFR (Cooper et al, IDEAL trial, NEJM 2010). A few years later, 2 trials examined the same thing in AKI and came up with contrasting results (Zarbock et al, ELAIN AKI, JAMA 2016 and Gaudry et al, AKIKI, NEJM 2016). That landed them in #10 and #3 in the top nephrology stories of 2016, though contrasting results meant one could cherry pick the data for clinical practice. A couple of years later, this question was examined again, in patients with sepsis, and starting dialysis early in critically ill patients was still found to be bad (Babar et al, IDEAL-ICU, NEJM 2018). IDEAL-ICU did snag #3 for top stories in 2018 though. If you put all these trials together, with a few other smaller trials, the total number still comes out to be just over 2000 (Gaudry et al, Lancet 2020). Size does matter, so this year STARRT-AKI (Bagshaw et al, NEJM 2020) should make all those itching to pull the trigger on early dialysis in AKI to cease fire. More than 3000 patients were randomized to starting dialysis when clinically indicated (standard) or earlier (accelerated strategy), on the basis of development of biochemical AKI (see the NephJC summary for the detailed discussion of eligibility and clinician ‘veto’). Not only was there no advantage of starting early, but more patients in the early arm remained dialysis dependent. Starting dialysis early in AKI is not helpful and it is harmful. Large trials are where biological plausibility and observational studies come to die. Don’t be the early bird, who likes to eat worms anyways?
2: SuPAR predicts AKI in COVID-19
With apologies to AA Milne
When suPAR was one,
It had just begun.
Telling us all that FSGS was won.
When it was two,
It was nearly new.
And lucky for us, was explaining CKD too.
When I was three,
I was hardly me.
Because now I was predicting mortality in diabetes you see.
When I was four,
I was not much more.
But predicted mortality in dialysis patients for sure.
When I was five,
I was just alive;
I was implicated in AKI, and therefore my use could thrive.
But now I am six,
I'm as clever as clever.
Because I predict AKI in Covid-19 infection,
And that’s going to be around for ever and ever
So I think I'll be six now.
At least until Top Nephrology Stories 2021.
1: DAPA-CKD- Dapagliflozin in diabetic and non-diabetic CKD
Flozination time. This has been a story long in the making - and the final chapter is still not written. From the days of phlorizin and green apples, the push from the FDA in 2008 for outcome trials following the Avandia adventures, to the successive sledgehammer of EMPAREG, CANVAS, and CREDENCE, is this year's debut of DAPA-CKD (Heerspink et al, NEJM 2020). It may even seem boring, yet another story of the flozins improving survival and preventing kidney failure. So what?
But have you seen a diabetic drug do that for non-diabetic patients? Closely following on the heels of two large heart failure trials (DAPA-HF: Mcmurray et al NEJM 2020 and EMPEROR-HF: Packer et al NEJM 2020), DAPA-CKD enrolled patients with diabetic kidney disease as well as patients who did have proteinuric CKD, but no diabetes. More than 4000 patients were recruited, and dapagliflozin had an astounding 39% relative risk reduction in the primary composite outcome. This was accompanied by a 34% reduction in requiring long-term dialysis, and a 31% reduction in all cause mortality. The effect was robust in patients who had diabetes, or who did not have diabetes. A supposed diabetes drug has benefits in non-diabetic CKD! Shout it from the rooftops! After the ARBy days of RENAAL, and IDNT, this is the first new therapy to conclusively demonstrate a benefit of clinical outcomes in nondiabetic CKD. Do you realize this was also the largest trial in IgA Nephropathy reported so far?
The bottomline is that SGLT2 inhibitors are very clearly not diabetes drugs anymore. They have repeatedly demonstrated robust results in cardiovascular and renal outcomes. We still need to understand how they work, and in whom else they might work (eg EMPA-KIDNEY which will also include non-proteinuric, non-diabetic kidney disease). That should not stop you from flozinating everyday. Whether you are a primary care provider or a specialist, a -flozin a day, will keep the doctor away.
Post by Matt Sparks, Joel Topf, Michelle Rheault, Tom Oates, Swapnil Hiremath