#NephJC Chat
Tuesday November 26 at 9 pm EDT
Wednesday November 27 at 9 pm GMT
Wednesday November 27 at 9 pm IST
Am J Kidney Dis 74 (4), 483-490 Oct 2019
Hypertonic Mannitol for the Prevention of Intradialytic Hypotension: A Randomized Controlled Trial
Mc Causland FR, Claggett B, Sabbisetti VS, Jarolim P, Waikar SS.
PMID: 31040088 Full Text at AJKD
Introduction
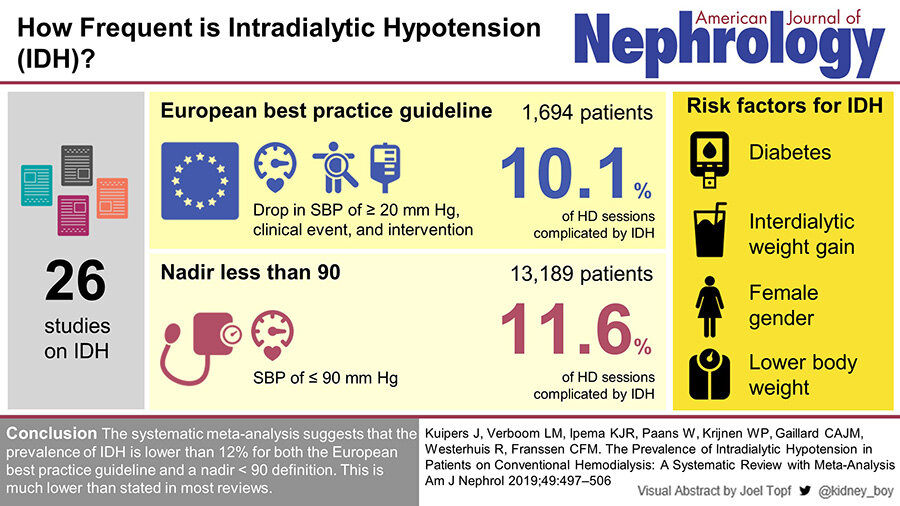
Volume management is an ever-present challenge in the care of patients on long term haemodialysis. The determination and subsequent achievement of optimal volume status involves balancing avoidance of interdialytic extracellulr volume (ECV) expansion with minimisation of intradialytic hypotension (IDH), now also being termed as hemodynamic instability during renal replacement therapy (HIRRT) in the setting of AKI. Its prevalence may be about 10% based on this systematic review:
There are variable definitions of IDH in the literature and guidelines; these include absolute decrease in systolic blood pressure, need to institute corrective measures, symptomatology or a change from pre-dialysis BP. IDH, particularly recurrent episodes, may result in multi-organ ischaemic insults and have been associated with end organ dysfunction including congestive cardiac failure and cognitive impairment, increased incidence of access failure, loss of residual kidney function and mortality.
Summary of underlying mechanisms that contribute to IDH/HIRRT. Source: Douvris et al
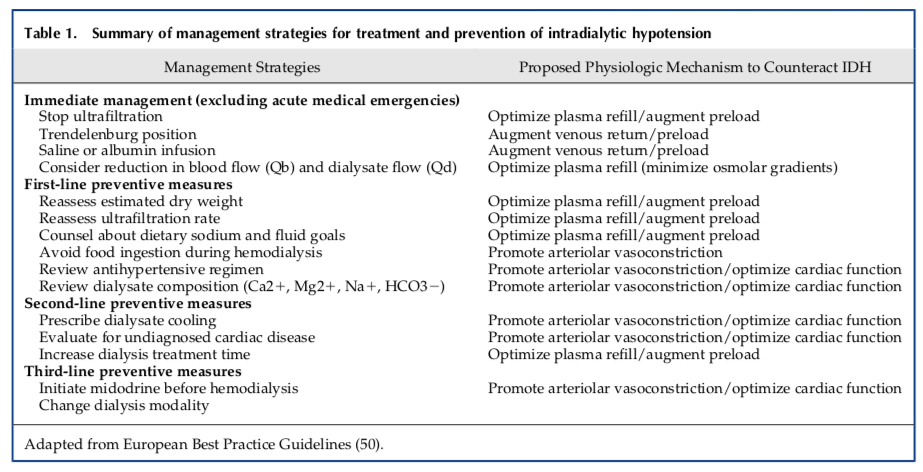
Although dialysis techniques have evolved and there is greater attention paid to IDH, the average age of dialysis patients and the proportion of those with conditions predisposing them to hypotensive episodes such as diabetes mellitus and heart failure has increased. Many treatment strategies are used daily in haemodialysis units across the globe; these are summarised in the table below
Source: Reeves and McCausland, cJASN 2018
Mannitol has been considered as a possible intervention to maintain adequate blood pressure; this is due to its oncotic effect as a result of increasing plasma osmolality. This study by McCausland et al was designed as a pilot study to assess the effect of mannitol on average intradialytic decline in systolic blood pressure (SBP) and the proportion of dialysis sessions complicated by IDH.
The Study
Methods
The trial is:
Double-blind
Placebo-controlled
Single-centre- Brigham and Women’s Hospital (BWH)
Inclusion and exclusion criteria
Adult patients initiated on HD for acute kidney injury or with progressive chronic kidney disease were considered eligible for enrolment. Since the supplement describes HD, presumably requiring CRRT was an exclusion too, apart from those described below.
The study excluded patients with
hyponatraemia as defined by preHD sodium <130mmol/L
acute MI, stroke/seizure within the last week
severe volume overload
unstable angina or ventricular arrhythmia
use of vasopressors
concomitant use of midodrine
Intervention
Patients were randomly assigned in a 1:1 ratio to receive 20% mannitol (0.25 g/kg/h; up to a maximum dose of 75 g per session) or placebo (equivalent volume of 0.9% saline solution; maximum 375 mL) .
Endpoints
The primary endpoint was intradialytic decline in SBP (predialysis SBP minus lowest intradialytic SBP). Secondary endpoint was IDH occurrence, defined as any drop of 20mm Hg or more from predialysis SBP.
Data collection
Plasma and urine laboratory analysis were collected including troponin T, plasma NT-proBNP, plasma and urine levels of kidney injury biomarkers. Demographic data, information regarding medication use and baseline laboratory data were collected before the first randomised visit. Additional information regarding dialysis prescription, BP measurements and reported adverse events were collected at each session.
Statistical Analysis
Continuous variables were recorded as mean ± standard deviation for normally distributed data or median with interquartile range for non-normally distributed data. variables were examined using frequency distribution and recorded as proportions. Comparisons were made using χ2 test. The difference in SBP decline between both groups was analysed by a repeated-measures missed event model using patient ID as a random effect. Linear regression models were fit to determine the effect of mannitol versus placebo on the difference in pre-HD biomarkers between HD1 and HD3, adjusted for pre-HD1 concentration. Interestingly enough, there is no mention of sample size analysis. Though this is meant as a pilot/feasibility trial to demonstrate the feasibility of implementing the protocol, these trials must be planned to provide thresholds for ‘success’ which would allow the next stage of a definitive trial to be performed.
Funding
No specific funding for the trial is mentioned, apart from the first author having received a K grant from NIDDK.
Results
52 patients requiring initiation of HD were enrolled between 2012 and 2016.
Figure 1 from McCausland et al, AJKD 2019
The groups were overall similar in terms of baseline characteristics except for pre HD SUN level which was significantly higher in the placebo arm. Table 1 does not mention how many patients were CKD or AKI at time of initiation of HD [update: but the text does mention 87% were for progression of CKD, so 13% were AKI presumably.}
Table 1 from McCausland et al, AJKD 2019
There was no significant difference in mean pre-dialysis HD, mean nadir intradialytic SBP and mean post-HD SBP between groups. From the unadjusted ITT analysis, the decline in (3.9mmHg) in the mannitol group was lower, however this did not reach significance when compared to the control group.
Figure 2 from McCausland et al, AJKD 2019
IDH occurred in 43% of placebo arm sessions vs 25% of mannitol arm sessions. Following analysis to allow for intrapatient correlation, mannitol use was associated with nominally lower odds of developing IDH (odds ratio 0.38; 95% confidence interval 0.14-1.00; p=0.05). A similar pattern was found when using the varying definitions for IDH.
Table 2 from McCausland et al, AJKD 2019
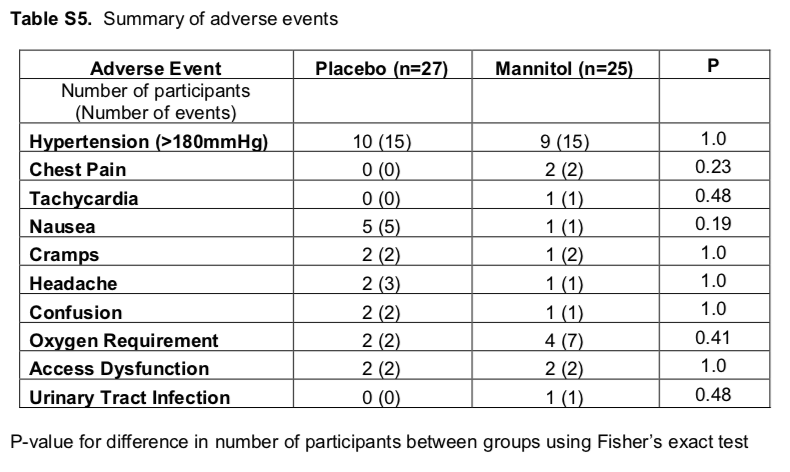
There was no significant difference in cardiac biomarker levels or adverse events between the treatment and placebo groups. There was no signal for elevations in levels of biomarkers of kidney injury; these levels appeared to increase from session to session in both groups.
18 participants (66.7%) in the placebo group and 17 (68%) in the mannitol group developed adverse events; most commonly hypertension and nausea.
Table S5 from McCausland et al, AJKD 2019
Discussion
This pilot study of incident HD patients showed no significant difference in intradialytic hypotension between the placebo and mannitol arms. Although there was a nominally lower frequency of IDH episodes in the treatment arm, this did not reach significance; a power calculation indicated approximately 194 patient would be needed to have adequate power for this outcome. Of note, there were no concerning safety signals in terms of biomarker levels, volume expansion or adverse events.
Strengths
Concealed randomisation
Double blinded
Independently prepared placebo
Weaknesses
Modest sample size
Inclusion of participants with AKI
Limited information regarding mannitol concentrations and intradialytic plasma osmolality
Timing of intradialytic symptoms not recorded
Timing of administration of antihypertensive agents, bioimpedance and measures of residual kidney function not recorded.
Whether there is any long-term benefit in the use of mannitol to prevent IDH remains unclear; larger studies are required to further evaluate this. What remains clear is that we need to continue to pursue research in this important area effecting haemodialysis patients globally. This pilot trial does provide some promising data which can inform the plan of a future definitive trial.
Summary by Laura Slattery,
Nephrology Specialist Registrar, Cork, Ireland
NSMC faculty