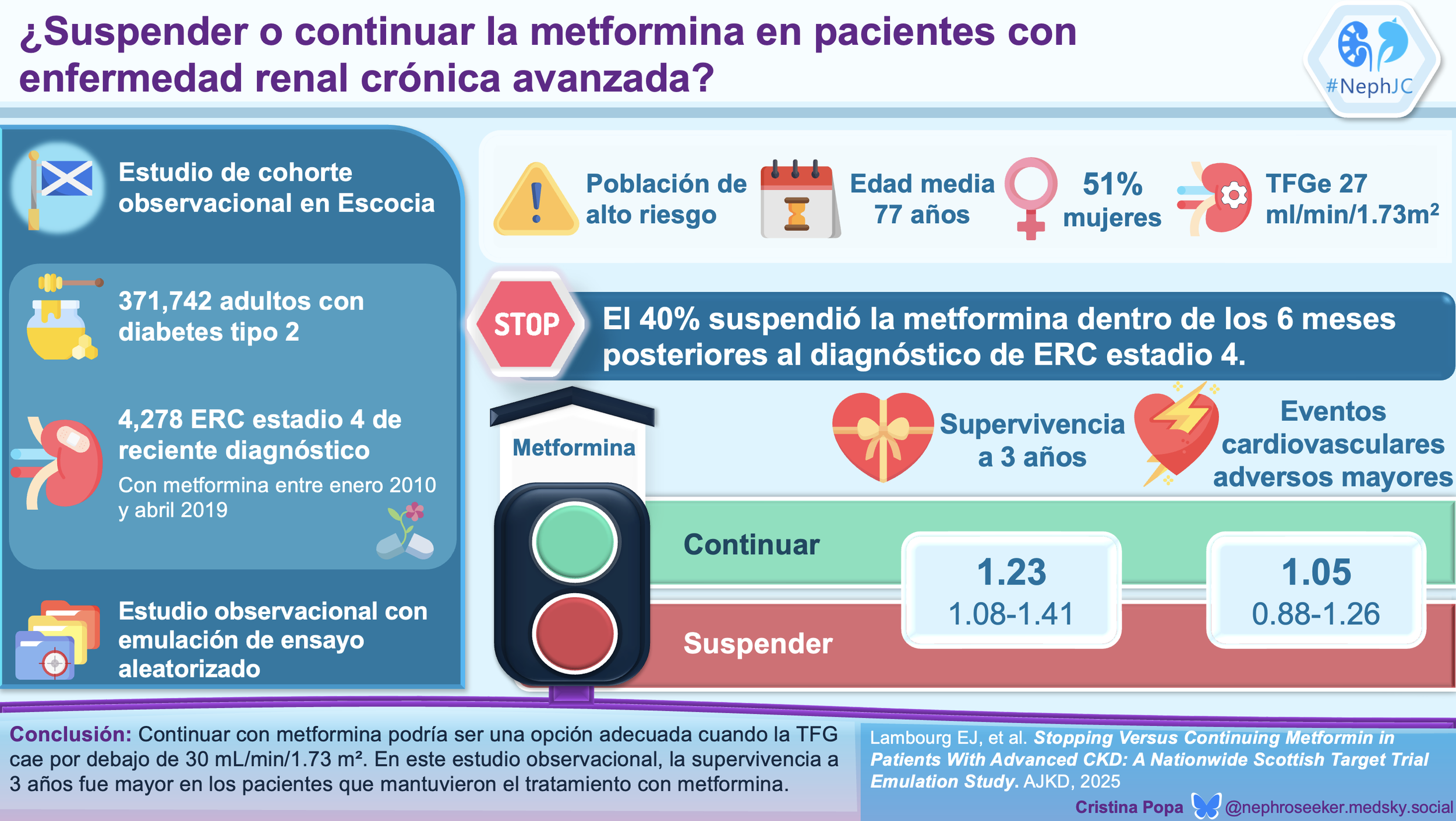
#NephJC Chat
Tuesday Jan 30 9 pm Eastern
Wednesday Jan 31 8 pm GMT, 12 noon Pacific
Diabetes Care. 2018 Jan 5. pii: dc172231. doi: 10.2337/dc17-2231. [Epub ahead of print]
Metformin Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease Stages 3A, 3B, or 4.
Lalau JD, Kajbaf F, Bennis Y, Hurtel-Lemaire AS, Belpaire F, De Broe ME.
PMID: 29305402
Introduction
**Disclaimer- this study discusses off label use of metformin in later stage CKD. The discussion below is based upon the above paper and does not constitute a change in the product labeling.
the current recommended use of metformin based upon kidney function is below.
From the FDA website link here.
Metformin is a mainstay of treatment in pre-diabetes and diabetes. However, it lives with the sins of its predecessor, phenformin. Phenformin was discovered in the 50's and became a popular in the 60's. By the 70's it began to lose ground as knowledge of it tendency to cause lactic acidosis became wide spread. Phenformin was banned from the US in 1977. It is still available in a handful of countries (Italy, Brazil, Uruguay, China, Poland, Greece and Portugal) and it sometimes slips into adulterated herbal medicines. Phenformin was estimated to cause 64 cases of lactic acdiosis per 100,000 patient-years.
Metformin, the second biguanide, always lived with questions about its safety in patients with Chronic Kidney Disease (CKD). When first approved in 1994, the FDA restricted it from patients with CKD, due to lactic acidosis concern since metformin is largely excreted by the kidneys. Metformin associated lactic acidosis (MALA) is a feared and deadly condition.
The FDA revised its warning on metformin and CKD in April 2016. Metformin remained contraindicated in patients with eGFR <30 ml/min/1.73m2. They advised that metformin not be started in patients with eGFR 30-45 ml/min/1.73m2, however if a patient was already on the drug and their eGFR decreased to this level, then the risks and benefits of metformin could be determined on a case-by-case basis.
Lalau and colleagues conducted three complementary studies to assess the risk of lactic acidosis in patients with CKD stages 3A, 3B and 4 taking metformin. As part of this they deduced CKD appropriate metformin dosing for these patients. Previous data for metformin use in CKD is limited to observational studies and was summarized in a 2017 systematic review article in the Annals of Internal Medicine by Crowley et al. This review included six observational studies in which metformin was used in CKD patients and found that metformin was beneficial. However, it is important to note, only one of the studies included patients with eGFR <45 ml/min/1.73m2.
Methods
This was an open-label, single center design, with three studies. Participants from the first study were invited to participate in the two subsequent studies.
Inclusion criteria
CKD 1-5 with stable renal function (<30% difference in eGFR within 3 months prior to study) in study 1.
Studies 2 and 3: CKD stages 3A, 3B and 4
Treatment with metformin or other antidiabetic drugs with HgbA1c > 6.5%
Lactate < 2.5mmol/L
Exclusion criteria
liver failure
pregnancy
breast feeding
Study 1: dose-finding study for patients with CKD 1-5
All patients, regardless of CKD stage, were given the same step-wise escalating doses of metformin. After one week of metformin at a fixed-dose, a 12-hour metformin trough was measured. Each step was followed by a 1 week washout period.
Moderately elevated: Metformin levels 2.5mg/L to 5mg/L
Clearly elevated: Metformin levels > 5mg/L
Study 2: 4-month metformin treatment to validate the optimal dose in patients with CKD 3A, 3B and 4
In this study, patients were given a fixed dose of metformin for 4 months based on stage of CKD. Dose of metformin was based on the results from Study 1.
Study 3: an assessment of metformin pharmacokinetics in patients with CKD 3A, 3B and 4
Again, patients with CKD 3A, 3B and 4 were included in this study. They received a fixed dose of metformin for one month with 500mg in the morning and evening dose based on their stage of CKD. Metformin levels at 0, 0.5, 1, 2, 4, 6, 8, 12 and 24 hours were drawn to capture the metformin pharmacokinetics.
Results
Study 1: Dose finding study
Figure 1 shows the data from study one and is a series of scatterplots of metformin concentration vs eGFR. At each dose there was a statistically significant inverse relationship between eGFR and the metformin concentration. Additionally, there was a significant difference between the slopes for the low dose compared to the high dose.
At the highest dose, 17 (out of 68 total patients) had a metformin concentration 2.5-5mg/L and 2 had a metformin level >5mg/L.
Study 2: Chronic, Dose-Adjusted Metformin Treatment
Figure 2 shows metformin concentrations at 1-month time intervals as a function of CKD stage. The peak metformin levels were 3.54 mg/L in plasma, below the FDA’s established elevated level of 5mg/L.
Figure 3 shows individual serum lactate at 1-month time intervals. Six patients of the 46 had lactate >2.5mmol/L. One patient had a lactate >5mmol/L however this was in the setting of a myocardial infarction. Not all of those patients are represented in the data because four of patients with elevated lactate were removed from the study because they changed their CKD stage more than twice during the study period (including the patient with serum lactate >5mmol/L). Seven total patients were excluded because change in CKD stage. There was no statistically significant relationship between lactate and plasma or erythrocyte concentrations of metformin.
Study 3: The pharmacokinetic study
Table 1 summarizes the results from the pharmacokinetic (PK) study. There was no statistically significant difference in any of the other PK parameters between the CKD stages. However, note that the terminal half-life of metformin increases in worsening CKD. Additionally, the maximum plasma concentration for all levels of CKD remain well below the FDA determined maximum safe metformin level of 5mg/L.
Discussion
This is the first of its kind to look at metformin use in patients with moderate to advanced CKD and appropriate metformin dosing in these patients. Based on the results, the study authors determined the following suggestions for clinical practice:
Suggest a daily dose of 1.5g in CKD 3A and 1g in CKD 3B
Assess eGFR every 6 months in CKD 3
Withdraw metformin in patients likely to experience acute kidney injury in the context of severe pathologies
Check lactate levels in fragile patients. Discontinue metformin if lactate >5mmol/L and observe level closely if lactate >2.5mmol/L
They do not provide dosing recommendations for off-label use of CKD 4. Instead, they suggest long-term prospective studies to assess the safety of metformin in these patients.
The study has the following limitations:
Single center, small sample size
No control group, including CKD 1-2 or patients with normal renal function. A control group would have been helpful to see if lactic acidosis is seen less frequently (or at all) in patients with mild CKD or normal renal function within the study population.
There is no established therapeutic level for metformin. The FDA has determined 5mg/L to be an elevated level, however no lower limit has been set for drug efficacy. This study does not recommend following metformin concentrations.
They were unable to determine if metformin therapy was associated with clinical outcomes. This is primarily due to the short nature of the study. They followed hgbA1c values of the patients and found no changes. A (much) longer study would be needed to demonstrate therapeutic benefit with metformin in patients with moderate to advanced CKD.
Many of the patients with elevated lactate levels changed CKD stage more than twice in the study period and so were excluded from the data and statistical evaluation.
Overall, this study provides valuable information regarding metformin dosing in patients with CKD 3, however there is not enough convincing data for patients with CKD 4. Future direction would include a longer prospective study. This study should be applauded for the in-depth pharmacokinetic parameters determined for patients with CKD, and the information obtained can be used to guide further studies. Since the study found the half-life of metformin increases with worsening renal failure, perhaps a study should be done different intervals between doses of metformin (ie longer time between doses depending on severity of CKD).
Will you be prescribing metformin in patients with moderate to advanced CKD? Join us Jan 30/31 to discuss!
Summary by Roopa Shah, Nephrology Fellow, Duke University