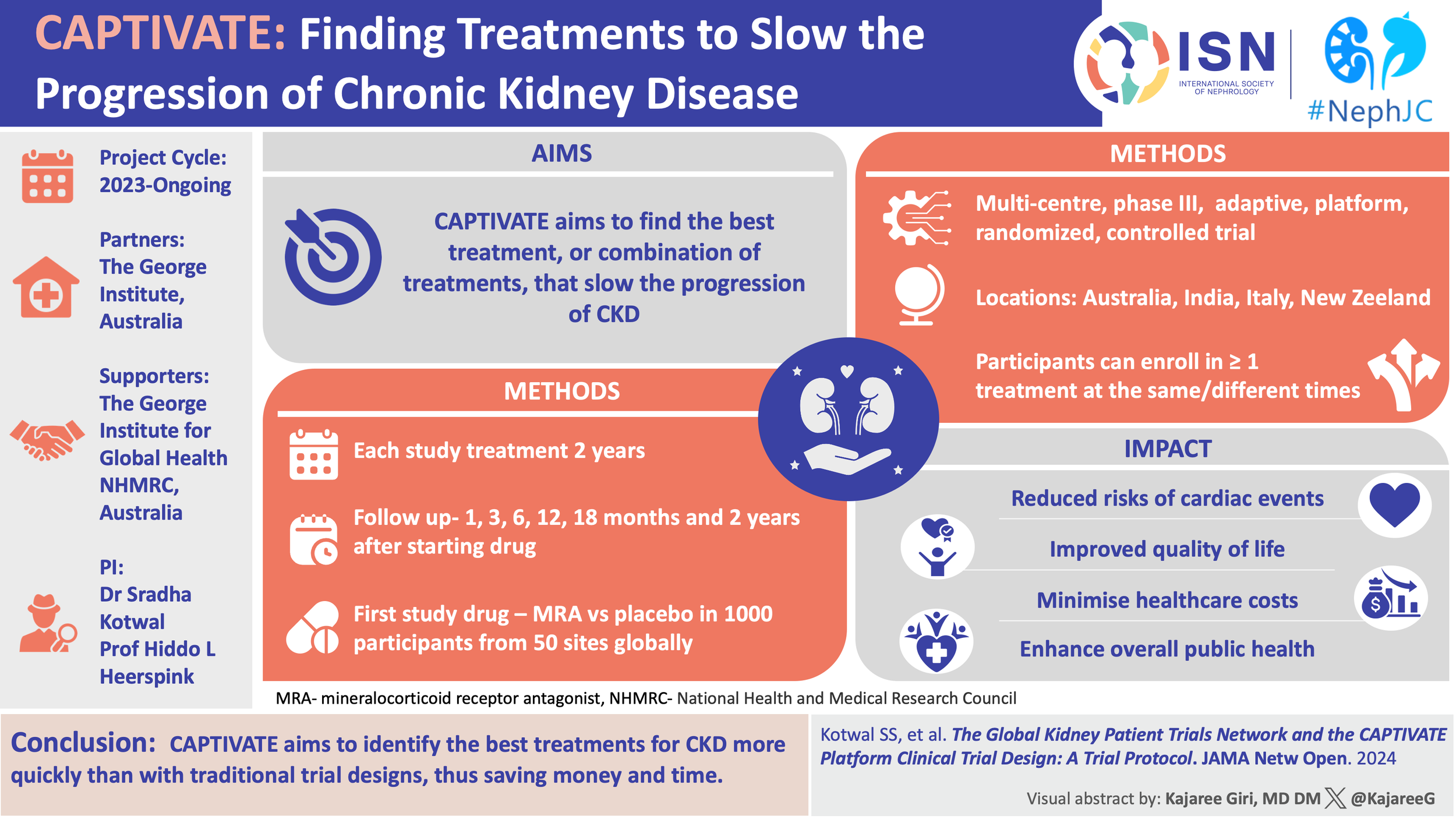
The story of RENALISM in COVID-19
Renalism (a portmanteau of therapeutic nihilism in renal conditions) was a term coined by Chertow and colleagues two decades ago in 2004 (Chertow et al, JASN 2004). They reported in an observational study of >57,000 elderly patients with acute MI that patients with CKD had >50% lower odds of undergoing coronary angiography than patients without CKD; and when angiography was performed, they were less likely to undergo revascularization than those without CKD. This focus on kidney function at the expense of the overall patient's condition has been coined “renalism”.
The philosopher Karl Marx once said “History repeats itself, first as tragedy, then as farce.”
Time and again we see “renalism” extend beyond procedural biases and lead to under-use of evidence-based medical therapies in patients with CKD. This is largely due to under-representation of patients with CKD in RCTs. Patients with stage 4 and 5 CKD with kidney failure have some of the highest rates of adverse outcomes (including premature mortality) but are often excluded in clinical trials, with even lower enrollment of those patients on dialysis or those who have received a kidney transplantation.
The COVID-19 pandemic has not been an exception to this. Morbidity and mortality from COVID-19 is higher in patients who are immunocompromised, including those with advanced CKD (stages 4 and 5) and those on dialysis. In 2020, Major and colleagues did a rapid review aimed to assess the CKD-related inclusion and exclusion criteria for COVID-19 trials (Major et al, JASN, 2020). They reported that patients with CKD were being excluded from almost half of all registered clinical trials for COVID-19. Subsequently studies evaluating nirmatrelvir/ritonavir (Paxlovid) have similarly excluded patients with advanced CKD, despite the relevance to this population. A perspective piece pushed the idea of prescribing Paxlovid in patients with advanced CKD and those on dialysis and even patients with kidney transplant, albeit with dose adjustments and pharmacy involvement (Hiremath et al, CJASN 2022; Hiremath CMAJ 2022).
Although vaccines are now available, there remains a need for other prophylactic agents against COVID-19 infection until vaccine use becomes widespread globally and effectiveness and durability is established, particularly in immunocompromised individuals, for whom vaccine responses may be suboptimal (Carr et al, KI Reports 2021). The PROphylaxis for paTiEnts at Risk of COVID-19 infecTion -V (PROTECT-V) is a platform trial designed out of necessity to test pre-exposure prophylactic drugs against SARS-CoV2 infection in vulnerable patient populations namely patients on dialysis, kidney transplant recipients and patients with vasculitis or glomerulonephritis on immunosuppressive agents who are all at high risk of COVID-19 and its complications.
Why did COVID-19 pandemic cause a resurrection of platform trial design?
In the last #Nephtrials edition we dove into the depths of the complex design of platform trials.
The COVID-19 pandemic essentially forced us to conduct clinical trials which are more patient-centric than ever before. The pandemic provided a unique research environment for which platform designs were particularly well-suited for rapid drug development and testing. COVID-19 perpetuated an explosion of platform trials: 58 COVID-19 platform trials were globally registered between January 2020 and May 2021 as opposed to 16 platform trials overall initiated between 2001 and 2009. One of the most famous is RECOVERY, which has been pivotal to many of the treatments we currently use (or do not use) in treating COVID-19. This speaks to the transformative changes in clinical trial designs!
Figure from Park et al The Lancet Global Health Vol 9 May 2021
Platform trials, also referred to as multi-arm, multi-stage (MAMS) design trials, are trials that evaluate several interventions against a common control group and can be perpetual. Trial learning is also perpetual as interventions enter and exit while conclusive evidence is being generated. Such nimbleness of adding and removing treatment arms and a common placebo arm has led to rapid evaluation and further approval of effective treatments against COVID-19, reduced trial costs and improved efficiency.
What is the significance of using repurposed drugs for COVID-19 prevention in PROTECT-V platform?
Drug repurposing means using a drug previously approved for one indication to target a new indication. Pandemics like COVID-19 are fertile grounds for drug repurposing as there is a sudden unmet public health need for rapid access to safe and effective therapies for a rather new disease, with no proven treatments available. The repurposed drugs can generally be made immediately available for use in clinical trials as they have known safety profiles at the licensed doses. For example, dexamethasone was repurposed in the RECOVERY trial and is estimated to have saved 1 million lives globally by March 2021 whereas tocilizumab, a repurposed drug for moderate to severe rheumatoid arthritis, is now licensed for treating hospitalized patients with moderate to severe COVID‐19 (Horby et al, NEJM 2021; Rosas et al, NEJM 2021) .
Greenblatt and colleagues examined the trajectory and sources of drug repurposing initiatives for COVID-19 (Greenblatt et al, Health Affairs 2023). Drug repurposing offers several advantages over de novo drug development:
Repurposed drugs are considered safer by avoiding the risk of exposing patients to a drug with unknown adverse effects.
Trial investigators may be able to use prior experience with a repurposed drug to expedite development and use for a new indication.
Price points of repurposed drugs are likely to be lower and more affordable and accessible to patients as compared to a new pharmaceutical innovation.
The PROTECT-V trial
Visual Abstract of the PROTECT-V trial from Deniise Arellano, ISN Social Media Team
PROTECT-V (Protocol available at Humphrey et al, Trials 2023) is by far the largest COVID-19 pre-exposure prophylaxis study of a repurposed agent to be conducted globally. Three separate agents have been included within the platform to date: intranasal niclosamide (a repurposed anthelmintic agent); inhaled and intranasal ciclesonide (a corticosteroid); and intravenous sotrovimab (a neutralizing monoclonal antibody directed against the spike protein of SARS-CoV-2). It is an example of the importance of global collaboration between charity funders, academia and industry partners; and demonstrates that COVID-19 clinical trials in vulnerable patient populations are possible and can be run safely and pragmatically in different healthcare systems. Participants have been enrolled in both the UK and India, reflecting different patient characteristics and risk factors, practice patterns, and health care systems.
As of February 2024, no biomedical intervention other than vaccines prevents COVID-19 disease. Previously, the FDA had authorized the use of the anti-SARS-CoV-2 monoclonal antibodies tixagevimab plus cilgavimab (Evusheld) as pre-exposure prophylaxis (PrEP) of COVID-19 in people who were not expected to mount an adequate immune response to COVID-19 vaccination and in people with COVID-19 vaccine contraindications. Due to the increased prevalence of Omicron subvariants that are not susceptible to tixagevimab plus cilgavimab, this combination is not currently authorized by the FDA for use as PrEP of COVID-19.
PROTECT-V platform is an international, multicentre study aiming to identify medications which protect vulnerable patients from COVID-19. The trial is enrolling participants who are at particularly high risk of COVID-19 and its complications due to being immunocompromised for any reason be that primary or secondary immunodeficiency.
Overall Inclusion criteria (specific arms have additional eligibility criteria)
Age over 18 and one of the following vulnerable patients populations
Dialysis - including in centre haemodialysis, home haemodialysis and peritoneal dialysis
Kidney transplant receiving at least one of the immunosuppressive medications listed below
Vasculitis or systemic lupus erythematosus (SLE) receiving at least one of the immunosuppressive medications listed below
Glomerulonephritis (includes prior histological confirmation of any of the following conditions - minimal change nephropathy, focal segmental glomerulosclerosis (FSGS), IgA nephropathy, primary membranous nephropathy, membranoproliferative glomerulonephritis or lupus nephritis) receiving at least one immunosuppressive medications.
These are vulnerable populations who are underrepresented in many existing clinical trials. The study opened to recruitment in February 2021. A parallel study protocol for Niclosamide arm was being conducted in India, sponsored by The George Institute for which recruitment commenced in February 2022.
Niclosamide arm (completed)
Niclosamide is a medication routinely used to treat tapeworm infections, which has demonstrated in vitro action against SARS-CoV2. It was hypothesized that this will disrupt SARS-CoV2 replication and penetration into cells. Niclosamide is typically taken as an oral tablet but PROTECT-V used a stable liquid formulation (UNI911) via a nasal spray in order to maximize the effect in the nasal lining where SARS-CoV2 initially predominantly replicates. 1,653 patients (1,233 from the UK and 420 from India) were randomized to either receive intranasal niclosamide or placebo for up to 36 weeks. Symptomatic COVID-19 infection was observed in 236 patients. The study did not meet its primary endpoint, as no difference was detected between risk of infection in the niclosamide and placebo groups (Preprint at Humphrey et al, SSRN, 2023). No major safety signals were reported in the study.
Ciclesonide arm
Ciclesonide is an inhaled corticosteroid (ICS) that has been shown to possess in vitro anti-SARS-CoV-2 activity. Ciclesonide inhibits in vitro SARS-CoV-2 replication in cultured human bronchial epithelial cells via a novel mechanism on non-structural protein 15 (NSP-15). ICS, particularly at higher doses, has been shown to reduce expression of ACE2 and TMPRSS2 receptors, which mediate infection of host respiratory epithelial cells. ICS have also been shown to inhibit in vivo production of IL-6, a key pro-inflammatory cytokine in COVID-19 and a major predictor of severe disease and poor outcomes. Inhaled ciclesonide and matched placebo, was added to the platform in early 2022 in the same renal patient population. Participants will be prescribed ciclesonide once daily, administered as follows:
Two puffs (320 μg) inhaled via mouth sequentially, One puff (160 μg) inhaled via nose.
Treatment duration will be 6-9 months or up to 28 days after COVID-19 diagnosis unless hospitalized.
Sotrovimab arm
Sotrovimab is a fully human IgG1κ monoclonal antibody (mAb) derived from the parental mAb S309, a potent neutralizing mAb directed against the spike protein of SARS-CoV-2. Sotrovimab is delivered as a single, one-off, intravenous infusion. The COMET-ICE study reported the efficacy of sotrovimab in the early treatment of COVID-19 (Gupta et al, NEJM 2021). It was used for the early treatment of those with COVID-19, but we do not yet know whether it can prevent infection. Enrolment began in August 2022 and is planned to recruit a minimum of 1760 participants to the sotrovimab arm in the UK.
There will be a single infusion of sotrovimab 2000mg administered at the beginning of the study.
Outcome Measures
Primary Outcome Measures
The primary outcome for PROTECT is confirmed symptomatic COVID-19 infection during treatment defined as the presence of both PCR confirmed SARS-CoV2 and one or more symptoms in keeping with COVID-19, including Respiratory, Constitutional and GI symptoms.
Secondary Outcome Measures
Time to confirmed SARS-Cov-2 infection from the date of randomisation including asymptomatic cases, Safety, All-cause mortality, Severity of COVID-19 disease.
Conclusion
PROTECT-V has already been pushing out results with the Niclosamide arm showing no difference in risk of infection between intervention and placebo group. The investigators should be commended for successfully conducting a global platform based RCT in this vulnerable and mostly ignored patient population. As we have seen so far with multiple ongoing platform trials like RENAL LIFE CYCLE, BEAT-Calci, PROTECT-V and others, the tide is changing and nephrology as a field is embracing a much needed innovation in clinical trial designs. Hopefully the PROTECT-V platform will eventually be successful in identifying a treatment for prevention of COVID-19 in this vulnerable patient population.
Manasi Bapat
Nephrologist at Kaiser Permanente,
Walnut Creek, California, USA.