#NephJC Chat
Tuesday November 27 at 9 pm Eastern
Wednesday November 28 at 8 pm GMT, 12 noon Pacific
J Clin Oncol. 2018 Sep 1;36(25):2612-2620. doi: 10.1200/JCO.2017.76.6691. Epub 2018 Jul 17.
Sirolimus for Secondary Prevention of Skin Cancer in Kidney Transplant Recipients: 5-Year Results.
Dantal J, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, Del Marmol V, Chatelet V, Dompmartin A, Kessler M, Serra A, Hofbauer GFL, Kamar N, Pouteil-Noble C, Kanitakis J, Roux A, Decullier E, Euvrard S; TUMORAPA Study Group.
PMID: 30016177 Free Full Text at Journal of Clinical Oncology
This will be a joint session with the Dermatology Journal Club
Introduction
Kidney transplantation is the light at the end of a very long, dark, tunnel for patients with end-stage kidney disease (ESKD). However, transplants come with their own baggage including increased risk of malignancies post-transplant. Some of the increase is from inhibition of immune surveillance, and some is from specific tumor development enhancement, from the calcineurin inhibitors. Skin cancers are especially common. Cutaneous squamous cell carcinomas increase the morbidity of these patients and the risk of subsequent episodes of squamous cell carcinoma can be as high as almost 80% in the three years following an index episode. (1, 2).
Use of sirolimus was associated with a 56% decreased risk of non-melanoma skin cancer
The mammalian target of Rapamycin inhibitors, ie, mTOR inhibitors, with Sirolimus (or Rapamycin named after its discovery on the Rapa Nui island amongst the Easter Islands, in 1972) as the poster child, are potent immunosuppressive agents - and possibly useful as calcineurin-sparing. The initial enthusiasm surrounding mTOR inhibitors as novel immunosuppressive agents was tempered with the discovery that sirolimus actually increased the risk of death. Still intriguing though, were their effects on post-transplant lymphoproliferative disease (PTLD) and other solid organ malignancies. mTOR inhibitors seem to have promising antitumor effects post-transplant as compared to calcineurin inhibitors (CNI). Even the aforementioned individual patient meta-analysis, which revealed increased mortality with sirolimus, reported decreased malignancy, especially a 56% decreased risk of non-melanoma skin cancer.
This week NephJC is teaming up with DermJC to discuss a prospective, randomized, long-term study in kidney transplant recipients on the conversion from CNI to a sirolimus-based immunosuppressive regimen for the secondary prevention of cutaneous squamous cell carcinomas. This study is an extension of the TUMORAPA studies which studied the effects of switching from CNI to sirolimus, as compared to remaining on CNI based therapy in kidney transplant recipients with at least one episode of cutaneous squamous cell carcinoma. The TUMORAPA studies were open label multicentre trials. TUMORAPA-1 included patients with a first cutaneous squamous cell cancer, and TUMORAPA-N included those with multiple squamous cell cancers. That study, which had a follow-up of two-years, showed that survival free of cutaneous squamous cell carcinoma, was significantly longer in the sirolimus group as compared to CNI-based controls. Interestingly, this difference remained significant for the patients with a single cutaneous squamous-cell carcinoma (hazard ratio, 0.03; 95% CI, 0.0 to 0.91) but not for those with more than one cutaneous squamous-cell carcinoma (hazard ratio, 0.67; 95% CI, 0.29 to 1.54). As we noted above, follow up was only 2 years - and whether this difference was persistent was unclear, hence this longer extension study. Also, adverse events and serious adverse events (eg pneumonitis) were far more common in the sirolimus group too, requiring discontunation in 15 of 64 pateints (23%).
The Study
Methods
This is a prospective, phase III, open-label, randomized controlled trial, which is an extension of the TUMORAPA-1 and TUMORAPA-N trials carried out in France.
Eligibility
It included patients who were
Calcineurin inhibitor-treated adult kidney transplant recipients
With stable kidney function
Who had developed at least one invasive post-transplant cutaneous squamous cell carcinoma
The major exclusion criteria (as reported in the original report) were
Multiorgan transplantations,
A history of organ rejection during the past 6 months,
Poor graft function (estimated glomerular filtration rate, <30 ml per minute, according to the Cockcroft–Gault formula; or 24-hour protein excretion over 1 gram),
Uncontrolled hyperlipidemia, hematologic or hepatic disorders, and retinoid treatment.
Intervention and Procedures
There was a 1:1 randomisation in the original study between continuation of the CNI therapy or switch to sirolimus and subsequent nephrology and dermatology follow-up for a period of 5 years as per a predefined schedule. Apart from all histologically proven skin tumors, various nephrology parameters like kidney function, 24-hour urine protein, and sirolimus and CNI drug levels were monitored. All patients had to be followed with dermatologic and nephrologic visits every 3 months for the first 2 years; then every 6 months until the 5-year mark (ie, 3 supplementary years) or the end of their participation in the study.
Outcomes
The primary endpoint was survival free of new cutaneous squamous cell carcinomas at 5 years. Secondary end points included time of onset of new cutaneous squamous cell carcinomas, occurrence of other skin and nonskin (ie, internal) malignancies, graft function and outcome, patient and graft survival, and the effect of the minimization strategies on recurrence. Additionally, crude hazard ratios were calculated looking at subgroups - patient with one or more than one cutaneous SCC at initial inclusion - using an accelerated failure-time model with interval censoring.
Analytic Plan
From the original 120 patients, the authors defined an ‘on-therapy’ population: ie including only the time period while patients were on the assigned treatment. As can be seen from figure 1 below, some patients swtched therapies: some from CNI to Sirolimus after developing skin cancer, and some from sirolimus to CNI after adverse events.
Role of Funding Source
The original TUMORAPA studies were sponsored by the Hospices Civils de Lyon, and grants were received from the French Ministry of Health, the French Society of Dermatology, and Pfizer (at that time Wyeth, maker of sirolimus). The study funders had no role in the study otherwise.
Results
From March 2004 to March 2009, 120 kidney transplant recipients with at least one cutaneous squamous cell carcinoma were recruited: 64 patients were randomised to the sirolimus arm and 56 were randomised to maintain their initial immunosuppressive treatment. At 2 years, 88 patients were available who were then enrolled into the extension study. 53 of them completed the 5-year follow-up with the treatment as per the initial randomization. The on-therapy population consisted of 120 randomly assigned patients who contributed a median follow-up duration of 39.7 months (34.9 and 46.2 months in the sirolimus and the calcineurin inhibitor groups, respectively). See figure 1 below for how the cohort evolved.
Figure 1 from Dantal et al, JCO, 2018
No table 1 is provided for baseline study characteristics - but presumably they would be the same as the original TUMORAPA studies, reproduced below:
Table 1 from Euvrard et al, NEJM 2012
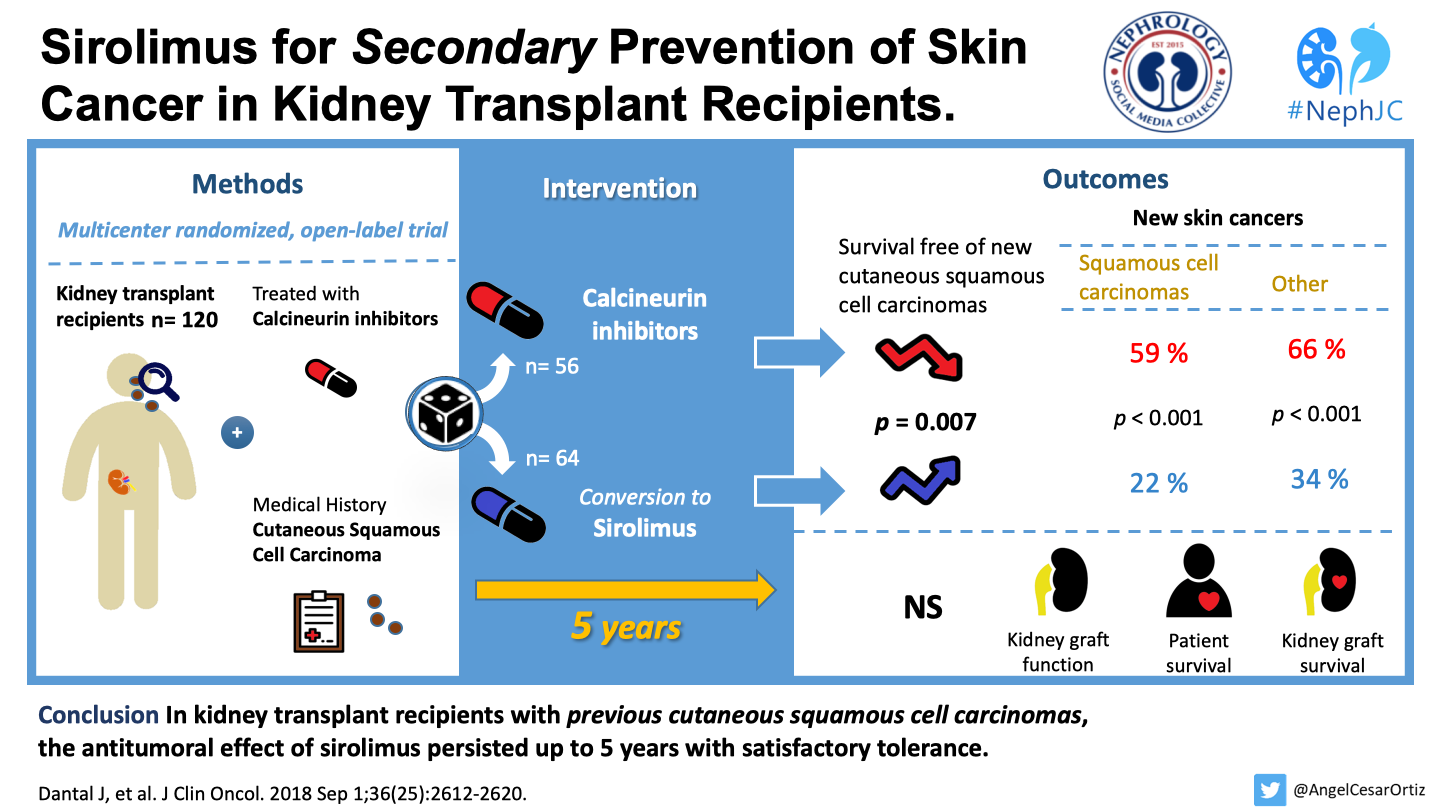
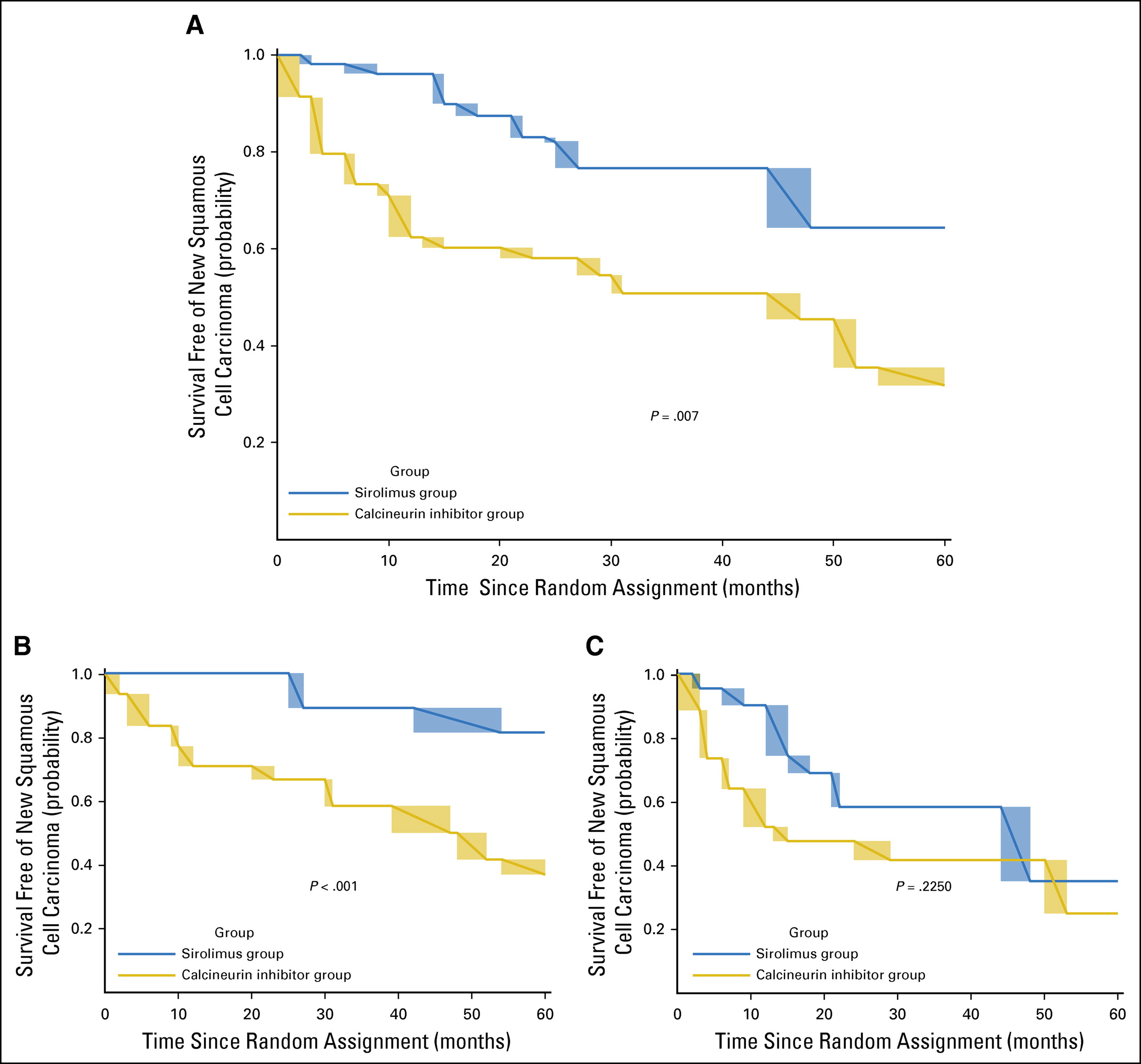
The primary outcome, time free of new cutaneous squamous cancer - was significantly longer in the sirolimus group than in the CNI therapy group (median time 23.5 months vs 12 months; p=0.007). See figure 2:
Figure 2 from Dantal et al, JCO 2018
As can be seen above, the difference was significant in the subgroup with a single prior squamous cell CA (panel 2B) but not those with more than 1 prior squamous cell CA (panel 2C) - same as the original TUMORAPA studies.
In the sirolimus group, the number of patients with new skin cancers (N = 14) was significantly lower compared with the calcineurin inhibitor group (N = 33), and also new basal cell CA (13 versus 21 patients, p = 0.044). However there was no difference in the occurrence of other non-skin malignancies amongst both the groups, though the numbers were small (N = 17).
Table 1 from Dantal et al, JCO 2018
Kidney graft function, patients, and graft survival were similar in both groups. There were 9 deaths - again not significantly different.
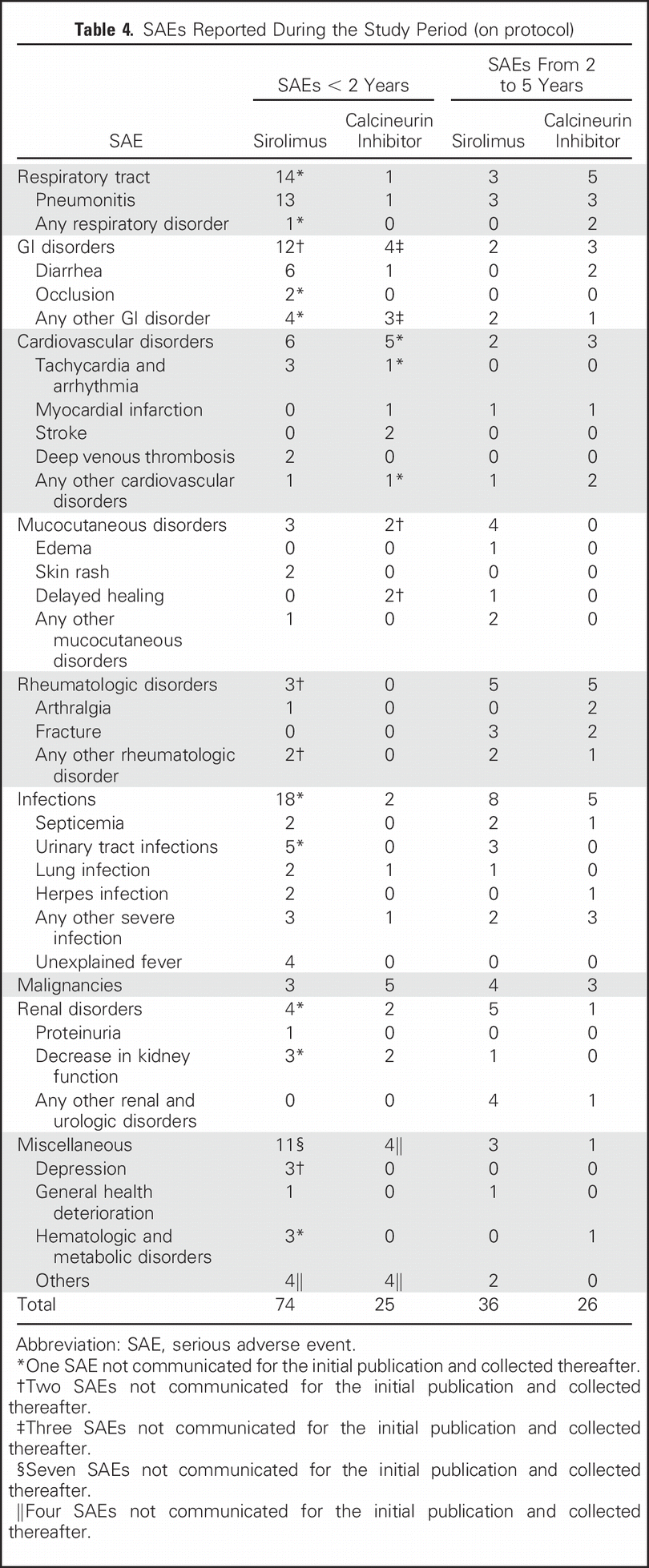
Beyond the 15 patients (23%) who discontinued sirolimus in the original 2 year follow up, 7 more patients (11%) had to discontinue sirolimus for adverse effects. See table 4 for the overall serious adverse events reported:
Table 4 from Dantal et al JCO 2018
Discussion
This study confirms what we know about sirolimus so far: it has a lot of adverse effects, and it lowers the risk of non-melanoma skin cancer.
Sirolimus does increase mortality however - so a blanket conversion of all patients is not the right strategy.
Also, the benefit was not seen in those with multiple prior squamous cell cancers - shown in the original trial, and seen again here, despite a larger number of events. Is there a window of opportunity after the first squamous cell CA when the switch from CNI to sirolimus has the most benefit?
Is the key aspect withdrawal of the CNI - or the addition of sirolimus? Could CNI withdrawal with some other CNI-sparing agent be just as useful (and help avoid the adverse effects of sirolimus)?
Conclusion
Conversion from CNI to sirolimus seems like a promising strategy in select patients. Given the serious risk of adverse events though, this requires careful weighing of risks and benefits - and shared decision making.
Summary prepared by Mayuri Trivedi,
Nephrologist, Hinduja Hospital, Mumbai
NSMC Intern, Class of 2018