#NephJC Chat
Tuesday, September 21 at 9 pm EDT
Wednesday, September 22 at 9 pm IST
Wednesday, September22 at 9 pm BST
Introduction
COVID-19 and Vaccines and mABs, Oh My!
As the delta variant continues to spread and the healthcare system and its providers remain under significant strain, a COVID-19 discussion is timely for this next installment of #NephJC. We will be discussing all things COVID-19 vaccine and monoclonal antibodies (mABs).
COVID-19 Vaccine
NephJC has posted up to date information on COVID vaccines throughout the pandemic which can be accessed here:
The American Society of Nephrology (ASN) also has extensive, up-to-date vaccine information:
American Society of Nephrology | Coronavirus Disease 2019 (COVID-19) - Home (asn-online.org)
And in breaking news, we cannot forget about the 9/20/2021 announcement on the promising Pfizer trial data in children age 5-12, giving hope to parents and healthcare providers that a vaccine for this age group may be available in the near future:
Monoclonal Antibodies (mABs) for COVID 19 Treatment and Prevention
The American Society of Nephrology (ASN) has compiled extensive information on mABs for COVID-19:
American Society of Nephrology | Coronavirus Disease 2019 (COVID-19) - Home (asn-online.org)
How do Monoclonal Antibodies Work for SARS-CoV-2?
SARS-CoV-2 infects cells via the receptor binding domain of the spike protein which attaches to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell. Anti-SARS-CoV-2 monoclonal antibodies used to treat COVID-19 target the spike protein to prevent host cell entry.
Monoclonal antibodies may also be used to treat COVID-19 by reducing inflammation rather than targeting SARS-CoV-2.
The FDA Emergency Use Authorization (EUA) for mABS
There are currently 3 Anti-SARS-CoV-2 mAB products with EUA status in the US for COVID-19:
Casirivimab plus imdevimab (REGN-COV2)- EUA issued November 21, 2020 with the latest update July 30, 2021
Bamlanivimab plus etesevimab – EUA issued February 9, 2021
Not authorized in US states, territories, or jurisdictions where variants are circulating that have reduced susceptibility to these products Bamlanivimab and Etesevimab Authorized States, Territories, and US Jurisdictions (fda.gov)Sotrovimab - EUA issued May 26, 2021
There is one mAB product with EUA status for COVID-19 that does not target SARS-CoV-2: Tocilizumab (ACTEMRA®) - EUA issued June, 24 2021
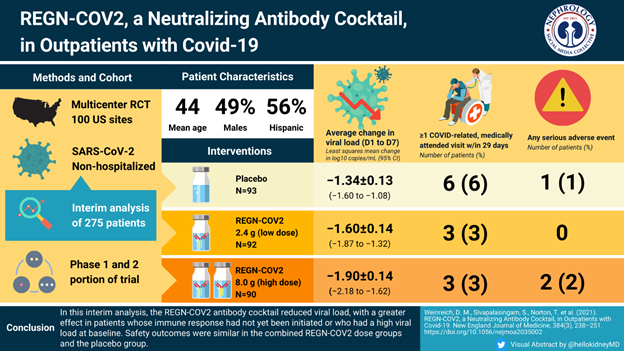
REGN-COV2 (Casirivimab plus imdevimab)
FDA Emergency Use Authorization for REGN-COV2 (as of 9/2021)
Regeneron EUA HCP Fact Sheet 09172021 (fda.gov)
REGEN-COV (casirivimab and imdevimab) supplied either as a co-formulated product or as individual vials to be administered together has been approved in the US under EUA for 2 indications: Treatment and post-exposure prophylaxis.
Treatment of mild to moderate COVID-19 in outpatient adult and pediatric patients who are at high risk of progression to severe disease
Eligible
Adult patient 18 years and older
Pediatric patient 12 years and older AND weight ≥ 40 kg
Positive SARS-CoV-2 test (PCR or antigen)
Mild to moderate COVID-19
Treatment within 10 days of symptom onset
Ineligible
Pediatric patients <12 years of age
Hospitalized for COVID-19
Oxygen therapy for COVID-19
Increase in baseline oxygen therapy due to COVID-19
Previous severe hypersensitivity reactions, including anaphylaxis, to REGEN-COV
Post-exposure prophylaxis of COVID-19 in adult and pediatric patients who are at high risk of progression to severe disease.
Eligible
Adult patient 18 years and older
Pediatric patient 12 years and older AND weight ≥ 40 kg
AND
Not fully vaccinated OR not expected to mount adequate immune response
AND
Exposed to close contact infected with SARS-CoV-2 (see CDC criteria COVID-19 Quarantine and Isolation | CDC) OR high risk of exposure to individual with SARS-CoV-2 (ex. Nursing home, prison)
Ineligible
Pediatric patients <12 years of age
Positive for SARS-CoV-2
SARS-CoV-2 variant with resistance to monoclonal antibodies
Previous severe hypersensitivity reactions, including anaphylaxis, to REGEN-COV
Criteria for Identifying High Risk Individuals at Risk for Progression to severe COVID-19
Older age (for example, age ≥65 years of age)
Obesity or being overweight (for example, BMI >25 kg/m2, or if age 12-17, have BMI ≥85th percentile for their age and gender)
Pregnancy
Chronic kidney disease
Diabetes
Immunosuppressive disease or immunosuppressive treatment
Cardiovascular disease (including congenital heart disease) or hypertension
Chronic lung diseases (for example, chronic obstructive pulmonary disease, asthma [moderate-to-severe], interstitial lung disease, cystic fibrosis and pulmonary hypertension)
Sickle cell disease
Neurodevelopmental disorders (for example, cerebral palsy) or other conditions that confer medical complexity (for example, genetic or metabolic syndromes and severe congenital anomalies)
Having a medical-related technological dependence (for example, tracheostomy, gastrostomy, or positive pressure ventilation (not related to COVID-19))
FDA Emergency Use Authorization for bamlanivimab and etesevimab (as of 9/2021)
Bamlanivimab and etesevimab administered together have been approved in the US under EUA for 2 indications: Treatment and post exposure prophylaxis.
Treatment of mild to moderate COVID-19 in outpatient adult and pediatric patients who are at high risk of progression to severe disease
Eligible
Adult patient 18 years and older
Pediatric patient 12 years and older AND weight ≥ 40 kg
Positive SARS-CoV-2 test (PCR or antigen)
Mild to moderate COVID-19
Treatment within 10 days of symptom onset
Ineligible
Pediatric patients <12 years of age
Hospitalized for COVID-19
Oxygen therapy for COVID-19
Increase in baseline oxygen therapy due to COVID-19
Previous severe hypersensitivity reactions, including anaphylaxis, to bamlanivimab and etesevimab
Post-exposure prophylaxis of COVID-19 in adult and pediatric patients who are at high risk of progression to severe disease.
Eligible
Adult patient 18 years and older
Pediatric patient 12 years and older AND weight ≥ 40 kg
AND
Not fully vaccinated OR not expected to mount adequate immune response
AND
Exposed to close contact infected with SARS-CoV-2 (see CDC criteria COVID-19 Quarantine and Isolation | CDC) OR high risk of exposure to individual with SARS-CoV-2 (ex. Nursing home, prison)
Ineligible
Pediatric patients <12 years of age
Positive for SARS-CoV-2
SARS-CoV-2 variant with resistance to monoclonal antibodies
Previous severe hypersensitivity reactions, including anaphylaxis, to bamlanivimab and etesevimab
Criteria for Identifying High Risk Individuals at Risk for Progression to severe COVID-19 (see above)
FDA Emergency Use Authorization for Sotrovimab (as of 9/2021)
GSK Sotrovimab Fact Sheet for HCP 07092021 (fda.gov)
Sotrovimab has been approved in the US under EUA for 1 indication: Treatment of COVID-19. Unlike REGEN-COV it is not authorized for use for post exposure prophylaxis.
Treatment of mild to moderate COVID-19 in outpatient adult and pediatric patients who are at high risk of progression to severe disease
Eligible
Adult patient 18 years and older
Pediatric patient 12 years and older AND weight ≥ 40 kg
Positive SARS-CoV-2 test (PCR or antigen)
Mild to moderate COVID-19
Treatment within 10 days of symptom onset
Ineligible
Pediatric patients <12 years of age
Hospitalized for COVID-19
Oxygen therapy for COVID-19
Increase in baseline oxygen therapy due to COVID-19
Criteria for Identifying High Risk Individuals at Risk for Progression to severe COVID-19 (See above)
FDA Emergency Use Authorization for Tocilizumab (ACTEMRA®) (as of 9/2021)
Genentech tocilizumab fact sheet for health care providers June 24 2021 (fda.gov)
Tocilizumab (ACTEMRA®) is an anti-interleukin-6 receptor monoclonal antibody approved in the US under EUA for treatment of COVID-19 in hospitalized patients with severe COVID-19.
Eligible
Adult patient 18 years and older
Pediatric patient 2 years and older
Positive SARS-CoV-2 test (PCR or antigen)
AND
Receiving systemic corticosteroids
AND
Require supplemental oxygen, non-invasive or invasive mechanical ventilation, or ECMO
Ineligible
Pediatric patients <2 years of age
Previous severe hypersensitivity reactions, including anaphylaxis, to Tocilizumab
Vaccination after mAB
Due to concern for possible blunted immune response, it is recommended that COVID-19 vaccines be administered ≥ 90 days after mAB administration.
Summary by
Missy Hanna, MD,MS
Pediatric Nephrologist,
University of Colorado
Visual Abstracts by
Carlo Trinidad, MD
Nephrologist, Manila, Philippines
NSMC Interns, Class of 2021