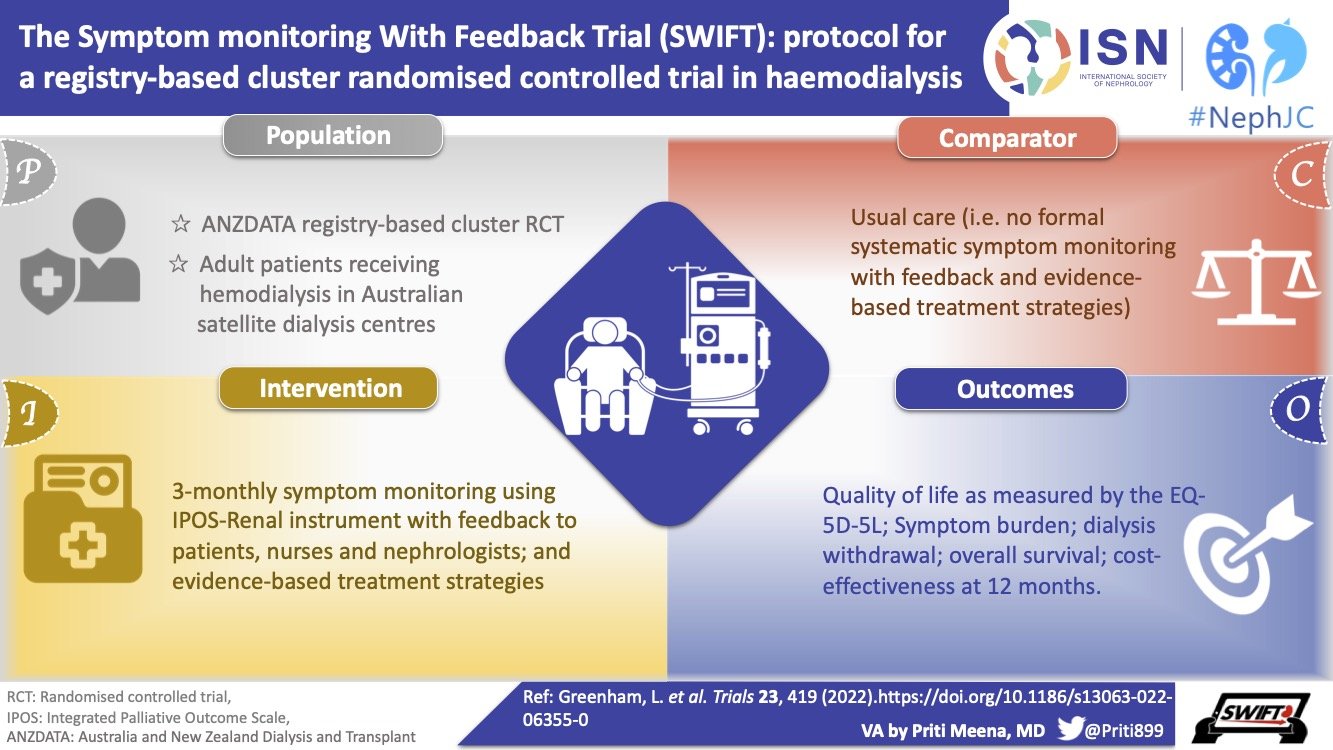
#NephTrials Chat
Tuesday Sep 28th 9 pm Eastern
After cluster RCTs and pragmatic trials, we will discuss the role of run-in periods and what we try to achieve by having them in clinical trials.
Heart Disease in Dialysis
Cardiovascular disease and mortality are the core outcomes most important for hemodialysis patients and increasingly studied in clinical trials among the dialysis patient population. Rightly so!
From Foley et al, AJKD Supp 1998, demonstrating the high mortalityin dialysis patients, mostly contributed by CV disease, compared to the general population (GP)
In the USRDS database, the single largest specific cause of death is attributed to arrhythmic mechanisms or sudden cardiac death (SCD). The overall best estimate is that SCD is responsible for approximately 27 percent of all-cause mortality in dialysis patients. Compared to the general population, patients on HD have a 5 – 30 fold increased risk of cardiovascular death, which accounts for about 50% of all-cause mortality. Treatments to improve CV outcomes in dialysis patients is the need of the hour. Most interventions have failed to show any effect, including prophylactic defibrillators (Wouter Jukema et al, ICD2 trial, Circulation 2019).
Figure from Tong et al, SONG HD outcomes initiative
In this edition of Nephtrials, the spotlight is on ACHIEVE trial: Aldosterone bloCkade for Health Improvement EValuation in End-stage Renal Disease (Clinical Trials protocol link). ACHIEVE aims to determine if spironolactone, a mineralocorticoid receptor antagonist (MRA), reduces death or hospitalization for heart failure and also to assess if it is well tolerated in patients that require dialysis.
We know that SCD is the most common cause of death in dialysis patients and is related to coronary atherosclerotic diseases, left ventricular hypertrophy and heart failure. Standard medical therapy for CAD and heart failure (HF) in dialysis patients includes a combination of medications that have been demonstrated to improve survival in patients with HF without renal failure, including beta blockers, ACE inhibitors or ARBs.
In 2012, a study (Dreschsler et al. European Heart Journal 2012) investigated the impact of high aldosterone and cortisol on cardiac and all-cause mortality in patients on maintenance HD and found that high aldosterone concentration in these patients is associated with significant increase in incidence of sudden cardiac death.
Although AT II is a key driver of aldosterone production, 30-50% of patients on ACEi/ARB have increased aldosterone - described as “Aldosterone Breakthrough” which is especially important in patients with ESRD given its association with SCD.
Mineralocorticoid Blockade in Heart Failure
Mineralocorticoid receptor (MR) blockade in heart failure patients has been shown to reduce sudden cardiac death and all-cause mortality. Direct effects of aldosterone and MR activation via mechanisms involving oxidative stress, inflammation and fibrosis occur both in heart and kidneys. (Check out the many mechanisms elaborated in Table S1 in a paper by Bauersachs and colleagues for preclinical evidence of the beneficial effects of MRAs in the heart and kidneys.)
In 3 landmark RCTs, MRA treatment in patients with HFrEF significantly reduced mortality and morbidity: RALES, EPHESUS and EMPHASIS-HF. Numerous small-scale clinical studies have demonstrated reductions in proteinuria or albuminuria with the addition of MRAs to ACE inhibitor/ARB therapy in patients with CKD and diabetic nephropathy independent of blood pressure.
Mineralocorticoid Blockade in Dialysis
In dialysis patients, two RCTs by Matsumoto et al and Lin at al did show reduction in the risk of both CV morbidity and death among HD patients with spironolactone use although the sample sizes were small, and the benefit these trials reported seemed implausibly large. Hence, large scale well-designed RCTs are needed for definitive guidance on spironolactone use in dialysis patients.
Not surprisingly, hyperkalemia is a significant adverse effect associated with use of MRAs. The reported incidence of serious hyperkalemia in the landmark MRA HF trials is 2 to 12%. The safety of MRAs in ESRD is a major concern and currently spironolactone and eplerenone are not approved by FDA for use in ESRD. Several smaller RCTs have investigated the role of MRAs in improving CV outcomes in dialysis patients with mixed results and safety concerns. Two of the most recent RCTs, SPin-D and MiREnDa, examined the effects of spironolactone in patients on chronic HD and showed relative safety regarding hyperkalemia but failed to show improvements in cardiac diastolic function over 36 weeks or LV mass index over 40 weeks.
Enter the ACHIEVE trial
A visual abstract of the design of ACHIEVE trial, created by Ahmed Akl @ISNeducation Social Media Team member and Fernanda Arce-Amaré, ISN Social Media Manager
The ACHIEVE study is a large, multicentre, double-blinded randomised controlled trial that examines the effect of spironolactone compared to placebo to determine whether spironolactone decreases cardiovascular death and heart failure in dialysis patients. ACHIEVE will recruit approximately 2750 participants across North America, Europe, UK, and the Asia Pacific Region. The primary outcome measure is CV Death or Hospitalization for Heart Failure. Secondary outcome measures include all cause death, cardiovascular death or hospitalization and hospitalization due to hyperkalemia. One of the key design elements of this trial is a pre-randomization active “run-in period” during which all patients will be given spironolactone to assess adherence and tolerance especially in respect to hyperkalemia. Patients with K > 6 and/or less than 80% adherence during active run-in period will be excluded from the trial and will not undergo randomization.
What is a Run-in Period in a Clinical trial?
The run-in period is a post recruitment and pre-randomization period in a trial usually lasting a few weeks to months during which all trial participants receive the same intervention: it could be active treatment, placebo, or no intervention. During this period, trial participants are assessed typically for adherence, intolerance, and optimize background therapy. At the end of the run-in period, participants with non-adherence or intolerance to the active treatment are excluded and do not undergo subsequent randomization.
Why do we care if a trial has a run-in period?
One of the most fundamental questions one asks during a journal club discussion is “Is this trial practice-changing and are the results relevant to the population I treat?” RCTs have goals that try to balance the tightrope of efficacy-effectiveness which we have discussed in our post on pragmatic trials. An intention-to-treat analysis in a trial with many non-adherent patients or high drop out rates can lead to significant underestimation of treatment effects, i.e the trial may fail to show efficacy. This is a significant waste of resources as RCTs are undeniably expensive endeavors. A run-in period is useful to exclude participants likely to be poorly adherent, to reduce risk of bias due to differential drop out or data collection, and to improve statistical power.
To understand whether the results of a particular trial apply to and benefit patients that one treats, it is of paramount importance to know who was excluded from the trial during the run in period and why. Laursen et al have done an observational study which concluded that reporting in run-in periods is often incomplete and suggest that all trials with a run-in period should report the number of excluded patients, reasons for exclusion, and baseline characteristics of excluded participants. For example, in the REPRISE trial for Tolvaptan in ADPKD, 126 patients withdrew before randomization and were reported as “run-in failure” but the authors have given a detailed description of reasons for withdrawal and provided baseline demographic and clinical characteristics of patients who withdrew from the trial.
Fig 1 from REPRISE trial, NEJM 2017
Other landmark nephrology trials with run-in periods are:
What are the advantages of a run-in period?
A run-in period is useful in trials with subgroup analysis for biomarker response and allows establishment of a comparator group. This is particularly useful in oncology trials for immunotherapy. In many of these trials, fewer than half the patients are expected to benefit from new treatment. Here, a run-in period is used to assess initial immunologic response to identify a subset of patients who will have a greater chance of eventual clinical response or for downregulation of target receptors to detect subsequent response to experimental drugs.
Run-in periods can be used to standardize and optimize a patient’s treatment prior to the intervention. For example, in the STOP-IgAN trial, all participants went through a 6-month run-in phase during which they received comprehensive supportive care that included RAASi to lower blood pressure to a target below 125/75 mm Hg, smoking and dietary counselling, and statins. Only those participants who had more than 0.75g per day protein excretion after the run-in period were eventually randomized between supportive care vs immunosuppressive therapy. Similarly in FIDELIO-DKD, the run-in period was used to ensure RAS blockade was optimized in all patients and potassium remained < 4.8 mmol/L with that to allow addition of finerenone (another MRA).
What are the different types of Run-in periods used in clinical trials?
Run-in trials that screen for placebo response
All participants receive single-blind placebo during run in period and patients who respond to placebo are excluded from subsequent randomization. This makes sense in trials testing drugs used for depression (if you respond to placebo, then you don't need the anti-depressant!)
Run-in periods that use trial drugs to screen for clinical response
Eg: In the US Carvedilol Heart Failure Study (Packer et al, NEJM 1996), 67 patients were excluded because of adverse events of worsening heart failure and death during run-in period and authors emphasized caution while initiating carvedilol in patients with heart failure. Similarly, in the SONAR trial of atrasentan in diabetic nephropathy (NephJC summary), there was a run-in period of optimizing underlying standard therapy, followed by an ‘enrichment’ period. This was an open label run-in (by another name) in which all participants received atrasentan, and those who either did not have a reduction in albuminuria, or had adverse effects of fluid retention were excluded. Thus, of 5100 who entered the enrichment period, only 2600 or so entered the randomized double-blind phase of the trial.
Figure 1 from SONAR trial, Lancet 2019
What are the potential problems with a trial with a run-in period?
Trials that use run-in periods to select patients on the basis of clinical response to the study drug may preclude a full assessment of both the therapeutic effectiveness and the safety of the drug when used in clinical practice.
In trials that use run-in periods to exclude non-adherent patients, comparisons between the two groups may still be valid but the estimates of treatment effects and tests of statistical significance will differ from what would have been observed without run in period.
In a published debate between Kenneth Schechtman and Milton Packer, Schechtman argues that by restricting randomization to participants deemed more likely to comply, run-in periods move the pendulum towards the goal of efficacy and away from effectiveness and public health paradigms. On the other hand, Packer has argued that the run-in period in fact mimics real world practice by selecting patients who will be adherent because in clinical practice, no patient is ever committed to long term therapy or efficacy assessments if they cannot tolerate or receive the new medication or return for follow up visits.
So, does the run in period in the ACHIEVE trial make sense?
I think it does. In ACHIEVE, participants with K more than 6 during run-in period will be excluded from eventual randomization between spironolactone and placebo. This is what we would practice in real world scenarios and nephrologists would be extremely hesitant to risk further hyperkalemia in patients with addition of an MRA in this group of patients. In case a benefit is seen with spironolactone in this trial, it would be important to understand that this effect would apply to those who tolerate spironolactone initially - and this is how the MRA initiation should be implemented. Without a run-in period, it may happen that, say, only 50% of the patients on spironolactone actually continue it, and one might get a spurious null effect.
With the completion of the ACHIEVE trial, we will be able to determine whether aldosterone blockade is safe and effective in reducing CV mortality in a subset of ESKD patients on dialysis. Let’s run and achieve the trial enrollment!