#NephJC Chat
Tuesday Nov 16 9 pm Eastern
Wednesday Nov 17 9 pm IST
Kidney Int. 2021 Oct;100(4):753-779. doi: 10.1016/j.kint.2021.05.015
Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases
Brad H Rovin 1, Sharon G Adler 2, Jonathan Barratt 3, Frank Bridoux 4, Kelly A Burdge 5, Tak Mao Chan 6, H Terence Cook 7, Fernando C Fervenza 8, Keisha L Gibson 9, Richard J Glassock 10, David R W Jayne 11, Vivekanand Jha 12, Adrian Liew 13, Zhi-Hong Liu 14, Juan M Mejía-Vilet 15, Carla M Nester 16, Jai Radhakrishnan 17, Elizabeth M Rave 18, Heather N Reich 19, Pierre Ronco 20, Jan-Stephan F Sanders 21, Sanjeev Sethi 22, Yusuke Suzuki 23, Sydney C W Tang 6, Vladimír Tesar 24, Marina Vivarelli 25, Jack F M Wetzels 26, Lyubov Lytvyn 27, Jonathan C Craig 28, David J Tunnicliffe 29, Martin Howell 29, Marcello A Tonelli 30, Michael Cheung 31, Amy Earley 31, Jürgen Floege 32
PMID: 34556300
KDIGO Website with full guideline coverage including
executive summary
full guidelines
data supplement
ISN-KDIGO webinars videos
Introduction
The Kidney Disease Improving Global Outcomes (KDIGO) initiative began in 2003 with the goal to improve outcomes of kidney disease worldwide. KDIGO achieves this by collaboratively developing and publishing clinical practice guidelines. The KDIGO guidelines for glomerular disease (non-diabetic) was first published in 2012. An updated KDIGO 2021 guidelines for GN was published in October 2021.The guideline is 281 pages long but the key points are compressed into a 27 page executive summary. The ISN has a two-part YouTube webinar which highlights the key differences from the previous version of the summary.
Methods
The full version and executive summary is broken down into 11 chapters. While the 2021 guidelines continue to use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, it uses practice points in addition to recommendations when systematic reviews and stronger level of evidence were not available but alternative options were illogical. These differences were discussed in an earlier NephJC summary. The guidelines are now incorporated into MAGICapp, an online platform which will facilitate faster revision as new evidence becomes available.
Chapter 1 discusses the general principles for the management of glomerular diseases. The subsequent chapters each focus on an individual disease, with chapters dedicated to IgA nephropathy/IgA vasculitis, membranous nephropathy, minimal change disease, FSGS, infection-related GN, anti-neutrophil cytoplasmic antibody (ANCA) vasculitis, lupus nephritis, and anti-glomerular basement membrane antibody GN. In addition, the guideline addresses a sub-type of complement-mediated disease for the first time.
Workgroups
The KDIGO work group consisted of multi-disciplinary international experts. Many of these experts have funding declarations from previous work with pharmaceutical companies, which are documented in the supplementary material.
Funding
KDIGO receives funding from many industries, see KDIGO Partners for full list. Ostensibly, the supporters are at arm’s length from the actual guideline development.
The Guidelines
Like all KDIGO guidelines, the guidelines are presented as
Guideline statement
Key Information including
Balance of benefits and harms
Quality of Evidence
Values and Preference
Resource use and cost
Considerations for implementation
Rationale
Research Recommendations
Chapter 1: General principles for the management of glomerular disease
While the general principles of managing GN remains same, there is a new recommendation to keep the BP below 120 mmHg systolic in adults and MAP’s below 50th percentile by ABPM in children. They also recommend using ACE inhibitors or ARB at maximally tolerated doses. This makes the GN guidelines consistent with the KDIGO 2021 BP guidelines (NephJC Summary). The kidney biopsy remains the “gold standard” for the diagnosis of glomerular disease but may not be needed in all cases. Also note the advice to measure proteinuria (not albuminuria, NephJC summary for why albuminuria might be better), and to use a protein:creatinine ratio from a planned 24 hour collection.
Management of proteinuria and hypertension reduction is a key aspect of treatment and practice points are highlighted in the table.
Chapter 2: Immunoglobulin A nephropathy (IgAN)/ Immunoglobulin A vasculitis (IgAV)
Given the results of the TESTING trials( NephJC Summary) and Stop-IgA studies (NephJC Summary), the initial mainstay of treatment in IgA nephropathy includes ACE inhibitor ARB along with supportive care and should be initiated for proteinuria >0.5g/d (Grade 1B). In those that do respond to supportive care after 90 days and are at high risk for CKD progression, a 6-month trial of glucocorticoids can be used.
Beyond steroids, the guidelines do not recommend other immunosuppressive agents except mycophenolate in the Chinese population and cyclophosphamide for rapidly proliferative GN. The caveat is that the routine recommendation does not apply to specific variant forms of IgA as highlighted in practice point 2.3.1.4. All children with IgAN and proteinuria >200 mg/d or PCR >200 mg/g (>0.2 g/g [20 mg/mmol]) should receive ACEi or ARB blockade, in addition to targeting optimal BP control and low salt diet.
Although there are not enough RCT’s in children showing superiority of immunosuppression in IgA over supportive care, the use of glucocorticoids and other agents is more widespread than in adults.
Glucocorticosteroids are not indicated for prevention of nephritis in IgAV and can be used in children with mild to moderate IgA vasculitis nephropathy (IgAVN).
Practice Point 2.3.2: Algorithm for the initial assessment and management of the patient with IgAN
Practice Point 2.3.1.4: Management of patients with IgAN who remain at high risk for progression after maximal supportive care
Chapter 3: Membranous Nephropathy
Membranous Nephropathy: Since the last 2012 guidelines, several new aspects to primary membranous nephropathy treatment have emerged including the role of anti-PLA2R, anti-THSD7A in guiding therapy. Important studies like GEMRITUX, MENTOR (NephJC Summary), and STARMEN (NephJC Summary) have led to new recommendations for the use of rituximab, calcineurin inhibitors, and cyclophosphamide.
In the 2021 KDIGO GN guidelines, a kidney biopsy is no longer necessary for the diagnosis of membranous nephropathy in the presence of M-type anti-Phospholipase A2 Receptor antibodies. The treatment of membranous nephropathy is based on risk stratification from low risk to very high risk for progression of CKD based on proteinuria, GFR, and serum albumin. Rituximab is indicated for moderate to high risk membranous nephropathy. Calcineurin inhibitors are not recommended as monotherapy but in conjunction with glucocorticosteroids or as adjunct with rituximab. Cyclophosphamide with glucocorticosteroids are recommended for high risk to very high risk diseases and those who are treatment resistant. Anti-PLA2R antibody levels should be used to guide therapy. Providers should determine if PLA2Rab are present prior to transplantation.
Chapter 4: Nephrotic Syndrome in Children
Minimal change nephrotic syndrome in children now favors use of oral glucocorticosteroids for a shorter duration of 8-12 weeks instead of 24 weeks. Based on multiple RCTs, there is recommendation to use low dose prednisone 0.5 mg/kg for a week during an episode of upper respiratory tract infection to prevent relapse in those with FRNS or SDNS. Steroid sparing agents like cyclophosphamide, mycophenolate mofetil, calcineurin inhibitor or rituximab can be used in those with steroid dependency or frequently relapsing course. Levamisole, although not available in many countries, can also be used in frequent relapses. Calcineurin inhibitor is recommended as the first line therapy in steroid resistant nephrotic syndrome patients to induce remission and subsequently can be switched to MMF to prevent long term CNI exposure and side effects.
Chapter 5: Minimal Change Disease in Adult
Nephrotic syndrome in adults warrants a kidney biopsy. High dose glucocorticosteroids constitute the mainstay, given for no more than 16 weeks with plan to taper steroids two weeks after patients achieve remission. Steroid sparing agents like MMF, CNI, cyclophosphamide and rituximab can be used in those with contraindications to steroids or frequently relapsing nephrotic syndrome.
Chapter 6: Focal Segmental Glomerulosclerosis (FSGS) in adults
A new classification system is proposed by these guidelines. FSGS of undetermined cause (FSGS-UC) was added to differentiate those patients with FSGS lesion on biopsy but who do not have any identifiable cause, while also not fitting the typical features of primary (previously called idiopathic) FSGS. Glucocorticoid therapy can be initiated in primary FSGS presenting with classic nephrotic syndrome. CNI is suggested as the next line of therapy if corticosteroid resistant. Those with higher likelihood for secondary FSGS should undergo supportive management. Genetic testing, when available, should be considered with treatment failure or in patients without nephrotic syndrome.
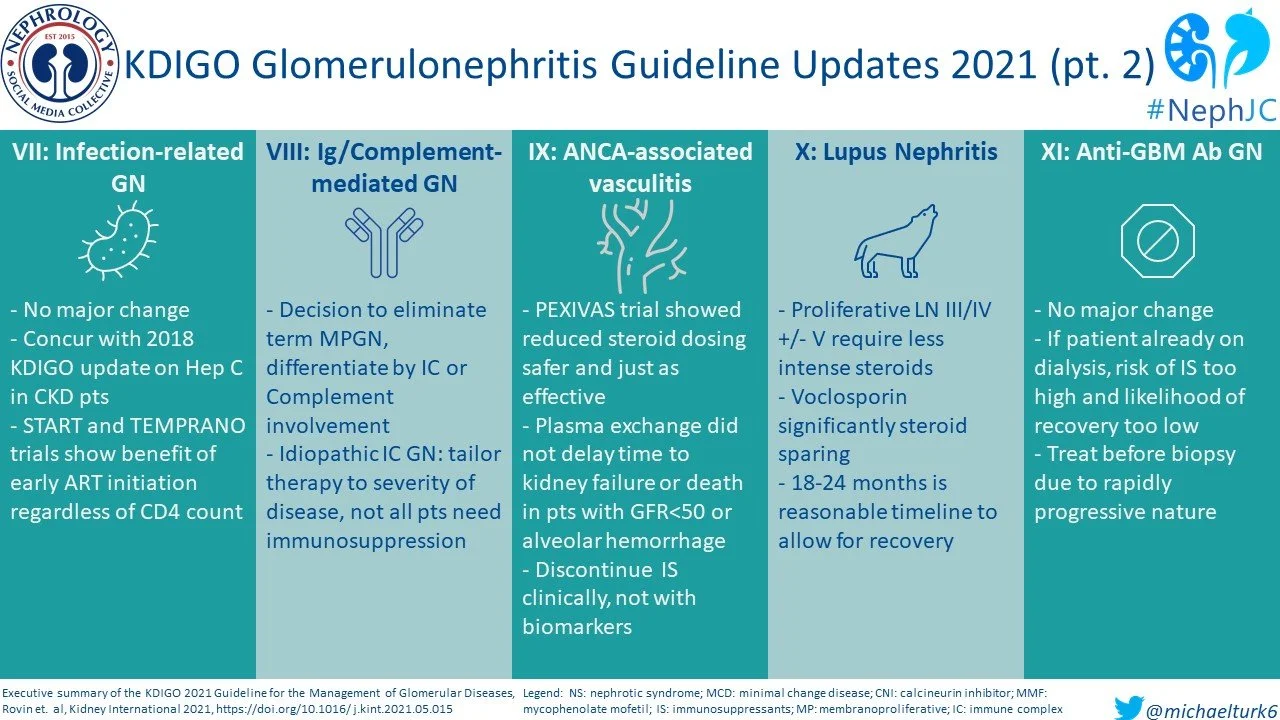
Chapter 7: Infection-related glomerulonephritis
Bacterial infection-related GN typical benefits from a biopsy and appropriate antibiotic therapy. IgA dominant infection-related GN is an entity seen in adults with risk factors like diabetes mellitus, hypertension, etc. and often requires dialysis. Hepatitis B and C infection-related GN should be treated per standard clinical practice. Immunosuppressive agents should be avoided to prevent accelerated viral replication.
Early initiation of antiretroviral therapy is recommended for HIV-associated nephropathy (HIVAN), regardless of CD4 counts. This is based on two large RCTs, the Strategic Timing of Antiretroviral Treatment (START) and Early Antiretroviral Treatment and/or Early Isoniazid Prophylaxis Against Tuberculosis in HIV-infected Adults (TEMPRANO).
Chapter 8: Immunoglobulin- and complement-mediated glomerular diseases with a membranoproliferative glomerulonephritis (MPGN) pattern of injury
Membranoproliferative GN(MPGN) disease is now replaced by more descriptive terminology as the membranoproliferative pattern is seen in histology in various settings with complement and/or immune complex dysregulation. The update from 2012 is in treatment for immune complex GN, where immunosuppressive therapy is now guided by severity of disease. MMF is suggested as the first line therapy in C3GN with eculizumab resereved for patients who fail to respond to MMF.
Chapter 9: Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis
The armamentarium of therapy in ANCA vasculitis now includes induction with rituximab or cyclophosphamide with glucocorticosteroids. Based on the recent Plasma Exchange and Glucocorticoids for the Treatment of ANCA-Associated Vasculitis (PEXIVAS) trial, a lower dosing of corticosteroids can be used. Following the results of this study, the role of therapeutic plasma exchange in ANCA disease presenting with severe kidney failure with/without need for dialysis is equivocal and KDIGO 2021 does not recommend its routine use unless there are associated anti-GBM antibodies. Maintenance therapy with rituximab or azathioprine plus low dose steroids are recommended for at least 18 months and should not be guided by biomarkers in ANCA, namely PR3 and MPO antibodies.
Chapter 10: Systemic Lupus Erythematosus
The management of lupus nephritis is likely expected to undergo changes as we obtain more systematic evidence on some novel therapies like voclosporin and belimumab. The AURORA trial(NephJC Summary) on oral voclosporin, a novel CNI demonstrated the multitargeted therapy with MMF and low dose steroids with voclosporin had better efficacy than MMF and steroids alone. It has not yet been compared to cyclophosphamide. As such, the initial induction therapy for lupus nephritis remains similar to the 2012 guideline with recommendation for lower cumulative dose of IV and oral prednisone and replacement of the NIH cyclophosphamide dosing regimen with Euro-lupus protocol. Biologics like rituximab are currently not recommended as first line but given results of recent RCTs, its use is suggested as a practice point. The Efficacy and Safety of Belimumab in Patients with Active Lupus Nephritis (BLISS-LN) trial (NephJC Summary) that was published in December 2020 demonstrated superior efficacy with addition of belimumab to MMF plus steroids than placebo. The primary end-point used in the trial was a more composite outcome defined as primary efficacy renal response (PERR) includes urine pCR <0.7, an eGFR decline not greater than 20% below baseline or at least eGFR 60 ml/min/1.73 m2 and no treatment failure.
Maintenance in lupus nephritis is with mycophenolate mofetil as the first line with individual consideration for azathioprine or CNI. The total duration of therapy remains largely unclear but need to weigh the risk benefit of side effects from immunosuppression versus concern for relapse. A repeat kidney biopsy could guide duration of immunosuppression in some cases. Newer studies have included class V membranous lupus nephritis but until more definitive evidence is available, the guidelines are unchanged from 2012 and recommends glucocorticoids with either MMF, azathioprine, cyclophosphamide or a CNI in those presenting with nephrotic syndrome.
Chapter 11: Anti-glomerular basement membrane (Anti-GBM) antibody glomerulonephritis
In a patient with RPGN, the diagnosis of anti-GBM disease should be made without delay in order to initiate treatment with corticosteroids, plasma exchange and cyclophosphamide. Plasma exchange should be performed until anti-GBM titers are no longer detectable. Conservative therapy is considered in those requiring dialysis, especially if they have >85% crescents on kidney biopsy.
Discussion
Maximizing supportive care with ACE inhibitor or angiotensin receptor blockers to control proteinuria and hypertension can go a long way in preventing CKD progression. Bombarding with big gun immunosuppressants may not always be the solution and likely cause more adverse effects without changing long term outcomes. Weigh benefits of immunosuppression with the adverse effects, especially infections, while making treatment decisions. Steroids still remain a winner as initial treatment in most GN, particularly in children. Rituximab has moved up the ladder in both membranous nephropathy and ANCA-associated vasculitis. The new kid on the block, voclosporin, will have to prove its full worth in future studies.
Conclusion
A lot has been done in the field of glomerular disease and a lot remains unanswered. We are seeing nephrology move forward on the backs of large multi-center clinical trials. The KDIGO 2021 provides a comprehensive and extensively reviewed guideline for the management of glomerulonephritis incorporating the results we have accumulated since the initial 2012 guideline. Hopefully this will be a living document, in a way we have not seen with prior KDIGO guidelines and we will see robust and frequent updates. As the nephrology community worldwide comes together to build knowledge, we can expect more refinement and stronger recommendations in the management of GN.
Summary by Shweta Shah
Pediatric Nephrologist
Texas Childrens Hospital
NSMC Intern, class of 2021
Cover image of a glomerulus under SEM, from Wellcome images, by David Gregory and Debbie Marshall, used under CC BY 4.0