#NephJC Chat
Tuesday June 20th 9 pm Eastern
Wednesday June 28th 8 pm BST 12 noon Pacific
N Engl J Med. 2017 Jun 12. doi: 10.1056/NEJMoa1611925. [Epub ahead of print]
Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group.
PMID: 28605608
Full slidesets (PPTx and PDF) from George Institute, Sydney
Introduction
We already discussed the results of the first SGLT-2 inhibitor (SGLT2i) to show demonstrable cardiovascular and renal benefits, the EMPA-REG-Renal study, which made a splash at Kidney Week 2015. Specifically, check out Joel's summary here on NephJC for details. They even got the ultimate nephrology red carpet treatment, as a NephMadness team, though they didn't snag the champions trophy to the chagrin of many. A few weeks ago, results from the second big SGLT2i trial were published, the Canagliflozin Cardiovascular Assessment Study (CANVAS). It is indeed important to establish Consistency, one of Bradford Hill's causation criteria,
Consistent findings observed by different persons in different places with different samples strengthens the likelihood of an effect.
Was EMPAREG was a flash in the pan, or is the beneficial effect of SGLT2i a class effect? Are there unforeseen adverse effects? There are some obvious differences between the individual agents from a pharmacological point of view (see table below), but are these clinically meaningful?
From T. Madaan et al. / European Journal of Pharmaceutical Sciences 93 (2016) 244–252
CANVAS, like EMPAREG before, was planned following what is euphemistically called the 2008 FDA 'guidance' for trials to explicitly examine cardiovascular safety in type 2 diabetes. This follows the experience with rosiglitazone, approved on the basis of hypoglycemic efficacy and then disapproved due to the fact the hypoglycemic benefit came with increased cardiovascular deaths. Though available in the US, rosiglitazone was withdrawn from Europe, UK, and other jurisdictions. Hence the main outcome measure in CANVAS is cardiovascular safety, with a non-inferiority design, closely following the FDA guidance.
Methods
Study Design:
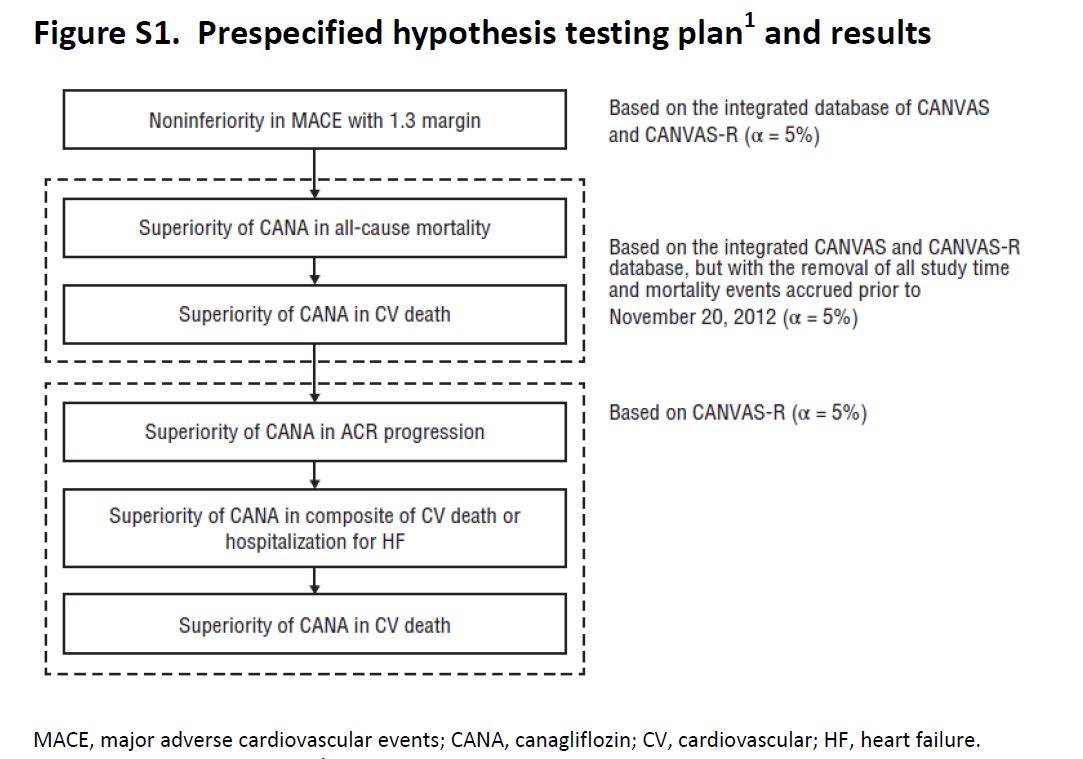
CANVAS was initiated in 2009, and on the basis of interim data from this trial, Canaglifozin managed to get FDA approval in 2013. The intent of CANVAS was that this would be a two-phase study: the first phase of the study with 4330 patients would examine the initial safety objectives for Canaglifozin. If the potential for cardiovascular protection was confirmed and initial safety criteria were met, the trial was supposed to enter a second phase that would recruit and follow an additional 14,000 individuals with the goal of demonstrating cardiovascular protection. But the sponsor elected to unblind the data while preparing materials for submission to the regulators. Accordingly, recruitment to the second phase of the study was shelved, and a separate large outcome trial, CANVAS-R, was planned and started in 2014. CANVAS-R recruited 5812 patients, and overall, the two sister trials were to be stopped once they accrued 688 cardiovascular events and the last participant had 78 weeks of exposure within the trial, which happened in Feb 2017. So how does one combine and analyse outcomes in such a set up? The trialists have an explicit hierarchy of outcomes (and hence the reader will notice at places where 95% CI are reported without a p value).
Figure S1, from CANVAS supplementary appendix, NEJM 2017
Among the differences between the two studies were the doses, in CANVAS a 1:1:1 ratio received canagliflozin at a dose of 300 mg, 100 mg, or 0 mg (matching placebo). In CANVAS-R patients were randomized in a 1:1 ratio to either receive 100 mg of canagliflozin, with an optional increase to 300 mg starting in week 13, or matching placebo. Note the 'placebo' is with a background of standard of care, including insulin and/or other hypoglycemic agents.
Population
Type 2 diabetes (glycated hemoglobin level, ≥7.0% and ≤10.5%)
Participants were required to have an estimated glomerular filtration rate (eGFR) at entry of more than 30 ml per minute per 1.73 m2 of body-surface area and to meet a range of other criteria (Table S1 for details)
Meet one of the follow two criteria:
≥30 years of age with a history of symptomatic atherosclerotic cardiovascular disease
50 years of age or older with two or more of the following CV risk factors:
Diabetes for at least 10 years,
Systolic blood pressure higher than 140 mm Hg while they were receiving one or more antihypertensive agents,
Current smoker
Microalbuminuria or macroalbuminuria
HDL cholesterol level of less than 1 mmol/L (38.7 mg/dL)
Outcomes
The primary outcome was a composite of
death from cardiovascular causes,
nonfatal myocardial infarction, or
nonfatal stroke.
Secondary outcomes planned for sequential conditional hypothesis testing were death from any cause, death from cardiovascular causes, progression of albuminuria, and the composite of death from cardiovascular causes and hospitalization for heart failure (see figure above for plan of sequential outcome analysis). If sequential testing was not significant for all the outcomes specified, the remaining outcomes were scheduled for assessment as exploratory variables in the integrated data set.
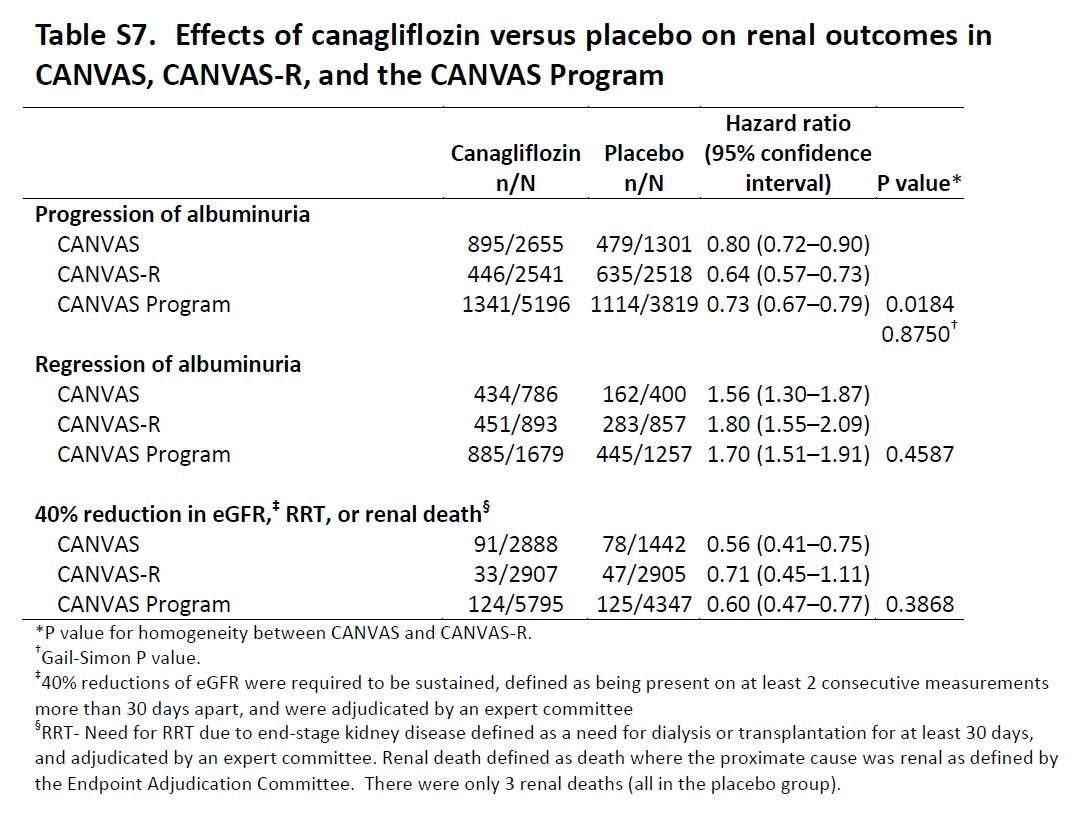
Renal Outcomes: Progression of albuminuria was defined as more than a 30% increase in albuminuria and a change from either normoalbuminuria to microalbuminuria or macroalbuminuria or from microalbuminuria to macroalbuminuria. The key prespecified exploratory renal outcomes were regression of albuminuria (using criteria comparable to those defined for category progression) and the renal composite comprising a 40% reduction in eGFR sustained for at least two consecutive measures, the need for renal-replacement therapy (dialysis or transplantation), or death from renal causes (defined as death with a proximate renal cause).
Role of Funding Source
"The trials were sponsored by Janssen Research and Development and were conducted as a collaboration between the sponsor, an academic steering committee, and an academic research organization, George Clinical. Analyses were carried out independently by the sponsor and George Clinical. ....The first draft of the manuscript was written by the first author, with all coauthors participating in subsequent revisions. MedErgy [NB: link added] provided medical writing support, funded by the sponsor. The authors, who had full access to the data and made the final decisions about the content of the manuscript, vouch for the accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol. The decision to submit the manuscript for publication was made jointly by all the authors."
Also note, 4 of the co-authors (N.E., W.S., G.L., M.D) are Janssen employees.
Results
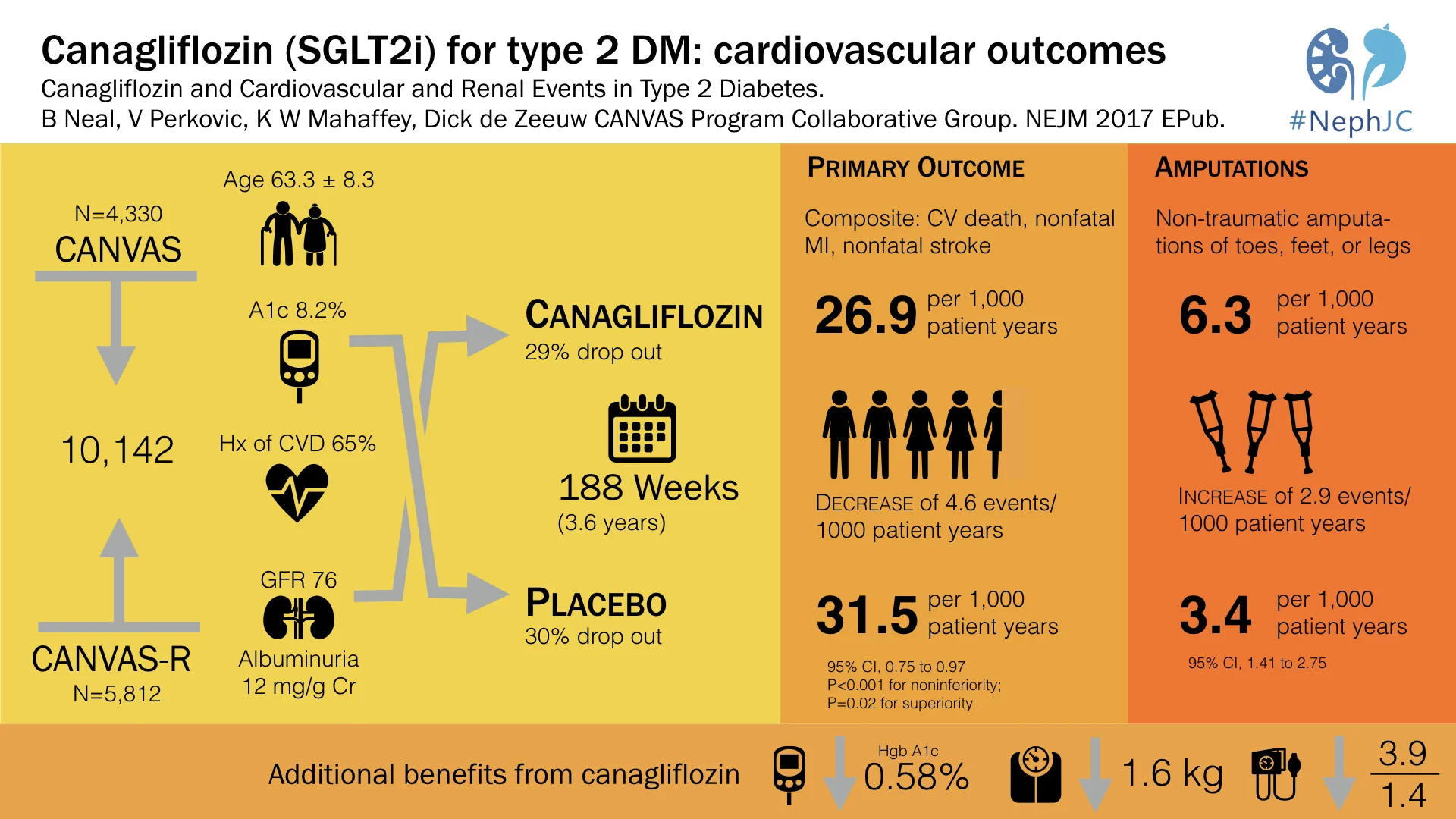
The overall N of participants included was thus 10,142 patients, as seen from the trial flow below.
from CANVAS study, NEJM 2017
Table 1 (and tables S4, S5) from the paper has the details on baseline characteristics: mean age ~ 63 years, ~ 60% men with mean duration of diabetes being about 13.5 years. Of interest to us, mean GFR was 76.5 mL/min, median ACR was 12.3 mg/g, 22.6% had microalbuminuria, 7.6% had macroalbuminuria, and 65.6% had a history of cardiovascular disease at baseline. See below for details of other diabetic and CV medications patients received. Interestingly, about 30% in both groups discontinued the study drug (see table S3 for reasons).
From CANVAS study, NEJM 2017
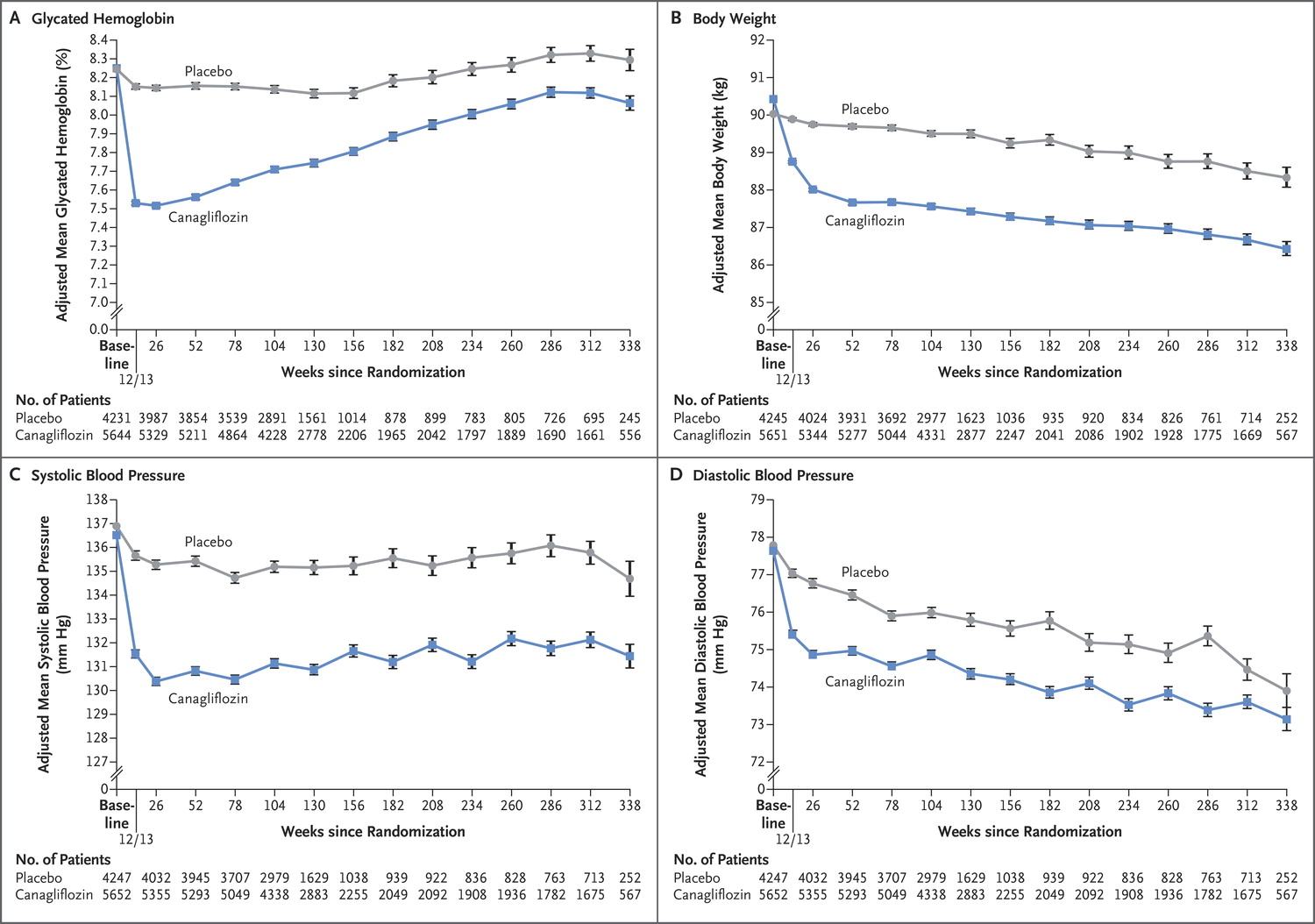
Predictably, canaglifozin reduced not only blood sugar (HbA1c) but also blood pressure, and weight, which should not come as a surprise to us after EMPA-REG.
Figure 1 from CANVAS, NEJM 2017
Like EMPA-REG, it did reduce overall mortality, but unlike EMPA-REG, the difference in CV specific mortality was not significant, hence p values for subsequent outcomes are not reported.
Figure 2 from CANVAS, NEJM 2017
From CANVAS study appendix; details of outcomes following the analytic plan (ie hierarchy of outcomes)
Thus, it was non-inferior to placebo with respect to MACE (with a margin of 1.3), it was also superior to placebo with respect to MACE, but it was not superior to placebo with respect to CV death.
Exploratory Renal Outcomes
Table S7 from CANVAS, NEJM 2017
Adverse Events
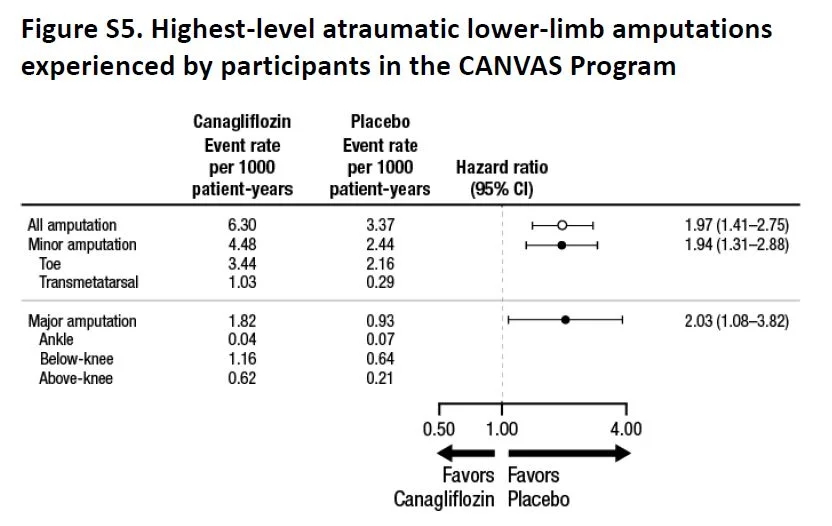
Overall, there were more events (as measured by event rate per 100 patient years, see below) in the placebo group, but most individual events seem either similar or higher in the canaglifozin group, so not sure how the numbers add up. Of specific interest, as expected, urogenital infections were higher in men and women, which were anticipated from the glycosuria. Similarly, volume depletion was higher, but AKI or hyperkalemia was not significantly different. And then there is the interesting new twist, more atraumatic amputations and also fractures.
Fig S5 from supp material, CANVAS NEJM 2017
Though the absolute number of fractures were higher in the toes/metatarsal level (71%), the relative risk of fractures at higher levels was also similarly higher with canaglifozin. This was not explained by previous history of amputation or peripheral vascular disease (table S8) so targeting those groups for close monitoring would not be especially helpful. Interestingly enough, though fractures were higher, the signal was in opposite directions in CANVAS and CANVAS-R (see interaction p values in table S9).
Discussion
The authors conclude that in patients with type 2 diabetes and established CV disease or at high risk for CV events, treatment with canagliflozin had CV benefit, and that in all three components of the primary outcome — death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke — showing point estimates of effect that suggested benefit, although the individual effects did not reach significance. There was also a lower risk of hospitalization for heart failure, progression of albuminuria, and substantive loss of kidney function than patients who received placebo, although on the basis of the prespecified hypothesis testing sequence these findings are not considered statistically significant.
Points for discussion
Is the CV benefit less than that seen with EMPA-REG, or is that just a function of power, and lower baseline CV risk?
What explains the greater risk of amputations and fractures? How big a concern is this? Is this a deal-breaker for canaglifozin (especially compared to empaglifozin)?
How do we translate the renal benefit (mostly driven by regression of proteinuria, and 40% reduction in GFR)?
Additional reading
Summary by Swapnil Hiremath