#NephJC Chat
Tuesday Nov 14th 9 pm Eastern
Wednesday Nov 15th 8 pm GMT, 12 noon Pacific
N Engl J Med. 2017 Nov 4. doi: 10.1056/NEJMoa1710030. [Epub ahead of print]
Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease.
Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators.
PMID: 29105594 Full Text at NEJM (will be free as online firstfor now)
This study was presented in the late-breaking session of the ASN meeting in New Orleans, and published in the NEJM the same day.
Background
Until recently, ADPKD for many nephrologists had become an untreatable, forgotten disease process, despite it being among the commonest causes of ESRD. Without so much as a sniff of an effective treatment, approximately 50% of ADPKD patients progressed to ESRD by 60 years of age.
Mutations in either PKD1 or PKD2 drive cystogenesis in the distal nephron and collecting duct epithelia. This is under the control of Vasopressin. For that reason, inhibition of vasopressin reduces cyst formation and preserves renal function in rodent models of ADPKD.
The previous CRISP study demonstrated the predictive value of increased total kidney volume (TKV) for progression to eGFR <30ml/min in ADPKD. Other work has shown correlation between cyst size and haematuria, hypertension and proteinuria. For these reasons, a therapeutic reduction in cyst size should benefit patients. This was borne out in the TEMPO 3:4 trial which demonstrated not only a reduction in TKV, but also a slower decline in eGFR, in participants treated with tolvaptan, albeit in a select group of patients with relatively preserved eGFR.
The Question – can TOLVAPTAN slow progression of advanced ADPKD ?
The previous TEMPO 3:4 trial showed that tolvaptan treatment of ADPKD patients, with a mean eGFR of 81ml/min, resulted in a yearly reduction in the rate of cyst growth by half, over 3 years. The study also showed a 30% relative reduction in the rate of serum creatinine increase over the same period. On this basis tolvaptan was approved for use in rather select cohorts of ADPKD patients in Europe and Canada, but not in America where the FDA requested further safety and tolerability data.
The current REPRISE study has responded to this request and has taken the TEMPO 3:4 work a step further by examining ADPKD patients with significantly reduced eGFR.
Trial design
Figure S1 from Torres et al, NEJM 2017
This is a multi-centre, double-blind, placebo-controlled randomized trial in adults aged 18-65 yr with ADPKD and established renal impairment, funded by Otsuka Pharmaceuticals. 2292 patients underwent a screening process, placebo run-in phase, then a tolvaptan run-in phase to ensure that they could tolerate the drug. This had been a previous concern arising from the TEMPO 3:4 trial due to drug side effects. Eventually 683 patients were randomized to tolvaptan and 687 patients were randomized to placebo and all were followed for 12 months. During the trial a reduction in tolvaptan dose was allowed.
The primary end point was change in creatinine-derived eGFR (CKD-EPI) based upon 3 measurements prior to randomisation and 3 measurements performed at the end of the study, with adjustment for exact time spent on treatment.
Safety measurements were conducted monthly and changes to hepatic enzymes were independently adjudicated.
Results
Primary & secondary endpoints
Loss of eGFR was reduced by tolvaptan from 3.61 ml/min/year to 2.34 ml/min/year (p<0.001). This 1.27 ml/min/year reduction compared to placebo was similar to that observed in the previous TEMPO 3:4 trial.
Encouragingly, 85% of patients remained on tolvaptan treatment at the end of the 12 month study period. The drop in eGFR with tolvaptan, was presumed to be a haemodynamic effect of the drug, and this effect reversed upon drug cessation.
Figure 2b from Torres et al, NEJM 2017
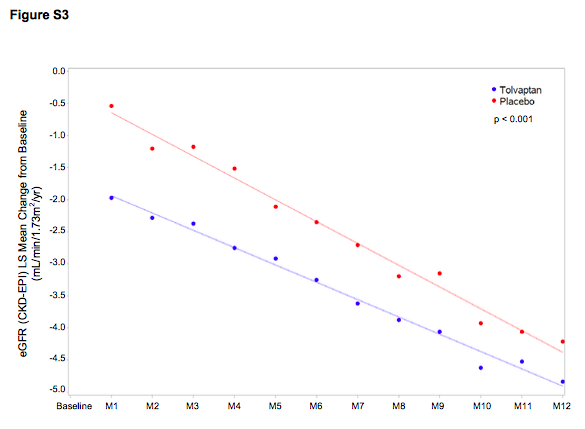
The mean slope of change in the eGFR between tolvaptan versus placebo showed a significant decrease in the rate of change with tolvaptan (difference 1.01 ml/min, p<0.001).
Figure S3 from Torres et al NEJM 2017
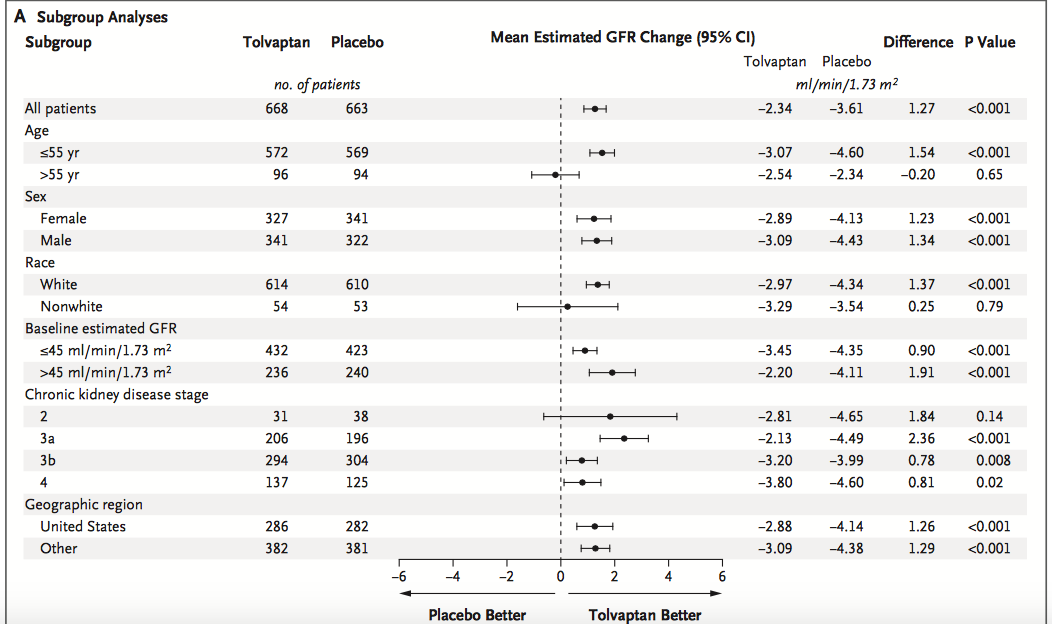
Within subgroup analysis, tolvaptan was superior to placebo even with more advanced disease, CKD4. Patients who were non-white, aged over 55yr or with CKD2 did not seem to benefit from tolvaptan although the small number of patients within these subgroups makes this conclusion less robust (and no p value for interaction is provided).
Figure 2a from Torres et al, NEJM 2017
The authors estimated the time to ESRD on tolvaptan versus placebo. Based on the observed rate of eGFR decline in their cohort, a patient with ADPKD with a starting eGFR of 41ml/min would reach ESRD after 9 years, versus 6 years if taking tolvaptan.
Other endpoints
The overall rate of adverse events was similar between tolvaptan and placebo groups during the double-blind phase of treatment but adverse events with tolvaptan were higher during the single-blind, run-in phase. The main reasons for discontinuation of drug related to aquaresis. Predictably, those taking tolvaptan had higher rates of polyuria, nocturia, thirst and fatigue.
Rises in the hepatic enzyme Alanine Aminotransferase to more than three times the upper limit occurred in 5.6% of Tolvaptan treated patients versus 1.2% of placebo treated patients (Hazard ratio 4.9). These changes were all reversible with stopping the tolvaptan.
Figure 3 from Torres et al NEJM 2017
What does REPRISE tell us that TEMPO 3:4 did not ?
The trials had different study populations and different endpoints. TEMPO 3:4 examined change in total kidney volume in early stage ADPKD (eGFR 81ml/min). On the other hand, REPRISE studied change in eGFR in established CKD (eGFR 41 ml/min) patients treated with tolvaptan, but did not examine kidney volume. Encouragingly, the two trials showed improvement with tolvaptan in their respective primary endpoints, both of which are important for tolvaptan patients.
Will REPRISE change the way we use Tolvaptan ?
Based upon TEMPO 3:4, nephrologists in Europe and Canada have used tolvaptan in a select group of ADPKD patients with mild CKD and rapidly enlarging cysts. The results of REPRISE suggest that we now could now consider tolvaptan use in patients with more advanced ADPKD, considerably broadening the use of the drug. Because REPRISE did not examine changes to TKV, the serial measurement of cysts, which has been a potential difficulty, may not be necessary.
Limitations
The TEMPO 3:4 and REPRISE studies have examined changes in ADPKD over relatively short time periods but ADPKD is a chronic disease. Replacement of normal renal tissue with cysts starts early in life, but patients exhibit a normal eGFR until years later, when they typically also experience flank pain, haematuria, urine infections, or renal colic. ESRD however takes years to develop. It is uncertain, whether tolvaptan will be safe, effective or tolerated over such extended periods of time. Encouragingly however, whilst TEMPO 3:4 initially showed an improvement if eGFR over 3 years, the TEMPO 4:4 follow-up study, in which patients were maintained for an additional 2 years on an open-label basis demonstrated that over the combined 5 year period the benefits of Tolvaptan on eGFR in ADPKD were retained.
Secondly, the side effects of tolvaptan will be a major problem for some patients. The REPRISE study pre-selected their participants based upon ability to tolerate the drug, but the trial still had a 9.5% dropout rate (versus 2.2% in the placebo group). In unselected ADPKD patients this dropout would presumably be substantially higher, as it was in the TEMPO 3:4 study (23% dropout on tolvaptan). Deranged liver function tests have also been detected in both the TEMPO 3:4 and REPRISE studies, albeit fully reversible on tolvaptan cessation. Longer term administration would require a monitoring and adherence programme to detect such events.
Finally, it is unknown whether tolvaptan could benefit the extrarenal manifestations of ADPKD such as intracranial aneuryms, valvular heart disease and non renal cysts.
Conclusions
Selected ADPKD patients with reduced eGFR may now benefit from tolvaptan treatment. Further studies will be required to show the long-term clinical outcomes, safety and drug tolerability profiles but hopefully tolvaptan will now be reconsidered for approval by the FDA for the treatment of ADPKD patients, who up until recently had no prospect of a drug therapy.
Summary by Ian Logan, Nephrologist, Newcastle on Tyne, UK